e-ISSN: 2319-9849
e-ISSN: 2319-9849
Metallo Therapy Limited, Wadi El Haj 13 Nazareth, Israel
Received date: 23/09/2016; Accepted date: 12/10/2016; Published date: 19/10/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
Background: Gold nano particles demonstrate size-related properties; their photonic and catalytic properties were found to be very attractive for materials applications as well as biomedical/biochemical. Methods to synthesis gold nano-particles were reported in previous studies, however, formation of uniform small gold nano particles and stabilizing these GNPs at protein containing solution is still challenging. Therefore, the main objective of this study is to develop a new simple and reproducible method to synthesize small gold nano particles and to stabilize them at protein containing solutions for possible medical uses such cancer imaging and therapy.
Results: We synthesized small gold nano particles with average diameter of ~ 3.5 nm as well we developed and synthesized a PEG–NAC conjugate to stabilize the GNPs at protein containing solutions.
Conclusions: Our new method to synthesize GNPs is successful and reproducible, further the new PEG-NAC conjugate that we developed is capable of binding the surface of the gold nano-particles and provides the GNPs enhanced solubility and improved stability particularly at protein containing solutions.
Gold nanoparticles, Synthesis, Polyethylene glycol, N-acetyl cysteine
Nano scale particles demonstrate size-related properties that differ significantly from those observed in fine particles or bulk materials; however, their performance stimulates a whole unit in terms of their transport and properties [1,2]. Gold nanoparticles (GNPs) are the most commonly studied form of nano scale particles mainly because their relative eases of synthesis and wide range of possible applications. Their photonic and catalytic properties were found to be very attractive for materials applications as well as biomedical/ biochemical [3-6].
Methods for synthesis of gold nano-particles have previously been reported: Turkevich method [7,8], Brust method [9], Perrault method [10], Martin method [11] and Navarro et al. method [12] where gold colloids are formed at low concentrations. Gold colloids; a typical lyophobic colloids bear large negative surface charge (surface potential of ~ 50 mV), therefore, they are stable only in very low-ionic-strength solutions. Gold colloids dispersed in water are thermodynamically unstable and usually require special stabilization.
The strong negative charge of the gold particle surface provides them strong adsorption interactions with high-molecularmass compounds [13]. Sulfur and gold atoms are known to form dative bonds. Alkane thiol linkers HS (CH2)nR (R=COOH, OH or SO3H; n=11 ± 22) are being used to achieve stronger attachment of bio-molecules to gold particles [14]. Interactions of these linkers with gold provide protective monolayer on the particle surface.
In recent years, synthetic polymers, such as polyethylene glycol (PEG), polyethyleneimine, polyvinylpyrrolidone, poly-(vinyl acetate), polyamidoamine (dendrimer), polydithiafulvene, chitosan, and the like, have found application in the synthesis of monodispersed colloidal gold (CG). Particles formed in the presence of these polymers are characterized by a higher size and shape uniformity. Unfortunately, the synthesis methods and stabilizers which have been used for producing stable gold nanoparticles did not provide the optimal gold nanoparticles for medical uses that need high stability of the GNPs solutions and high cellular uptake of the GNPs [15,16].
Mainly, possible future use of gold nano particles for treatment and diagnosis of health related conditions requires improved properties of the GNPs such as improved stability, improved solubility, reduced toxicity, enhanced bioavailability and improved pharmacokinetics. Hence, there is still a need in the art to develop new synthetic methods to produce stable concentrated GNPs solutions and new molecules to modify, protect the GNPs surface and to prevent their interaction with the environment.
Here we report a new simple and reproducible synthesis method of stable aqueous dispersion of surface-modified gold nano particles. Our new method is based on the use of micro emulsion to guarantees a soft reduction process by reducing the interaction between the reducing agent and the gold complex, further, the use of micro emulsion to control particle properties such as size, geometry, morphology, homogeneity and surface area. In addition, we introduce a novel polyethylene glycol (PEG)-Nacetyl cysteine (NAC) conjugate (PEG-NAC) that binds the surface of the gold nanoparticles via the thiol group of the NAC intended to provide the GNPs enhanced solubility and improved stability particularly at protein containing solutions.
It has been demonstrated in previous works that following the exposure of GNPs to protein containing solutions; the GNPs interact with the thiol groups of the cysteine residues exposed on protein surfaces as a result micro aggregates appears and GNPs lose their Nano characteristics [17]. PEG-NAC conjugates can attach the surface of the GNPs via the free thiol group of the NAC. We believe that PEG-NAC conjugate can possibly hinder protein-gold interaction by two mechanisms: by blocking binding sites at the GNP surface, and by interfering ligand exchange process that usually occurs between the original coating layer and the nearby proteins. Accordingly, the PEG-NAC modified GNPs could possibly demonstrate higher levels of stability at protein containing solutions as well as at physiological solutions more than other modified GNPs such NAC modified GNPs and thiolated amino acids modified GNPs.
The novel PEG-NAC surface-modified heavy metal nanoparticles possibly can exhibit improved solubility in water (i.e. being highly hydrophilic); improved stability (stable for a long period and stable at physiological conditions); reduced toxicity; improved bioavailability; improved pharmacokinetic properties (higher half-life in blood); easy and cost effective to produce (even at larger industrial scale).
Synthesis of gold nano-particles
All chemicals were purchased at Sigma Aldrich. 3.75 ml of a fatty acids mixture including 65% oleic acid, 18 % linoleic acid and 16 % palmitic acid, 200 mg NaOH and 15 ml ethanol were added to 30 ml water. The mixture was allowed to stir for 5 minutes on a magnetic stirrer at room temperature to form micro emulsion. 50 mg of HAuCl4 was added while stirring, resulting in a yellowish solution. To achieve reduction, 5 ml of 0.05M ascorbic acid was added slowly to the mixture. Change of the solution color to red-wine indicated that the gold reduction process was completed.
Subsequently, 124 mg of N-acetyl cysteine was slowly added to the mixture. The mixture was stirred for additional 30 min allowing the attachment of the NAC to the surface of the GNPs. The pH of the solution was then adjusted slowly to 9 using 2M of NaOH. The resulting mixture was further stirred for at least an hour at room temperature. 20 ml of Hexane was added to the stirred mixture and left for an additional one hour.
PH of the mixture was adjusted to 7 using 12 M HCl solution. The mixture was separated into two phases, an organic phase and an aqueous phase where the gold nano particles are dispersed in. Aqueous phase was dried under vacuum at temperature of 50°C to remove the water and traces of organic solvents. The dried powder comprising surface-modified gold nanoparticles was dissolved in 5ml water; yielding an aqueous dispersion of NAC modified gold nanoparticles. Finally, the aqueous dispersion was dialyzed using dialysis tube to remove unwanted materials.
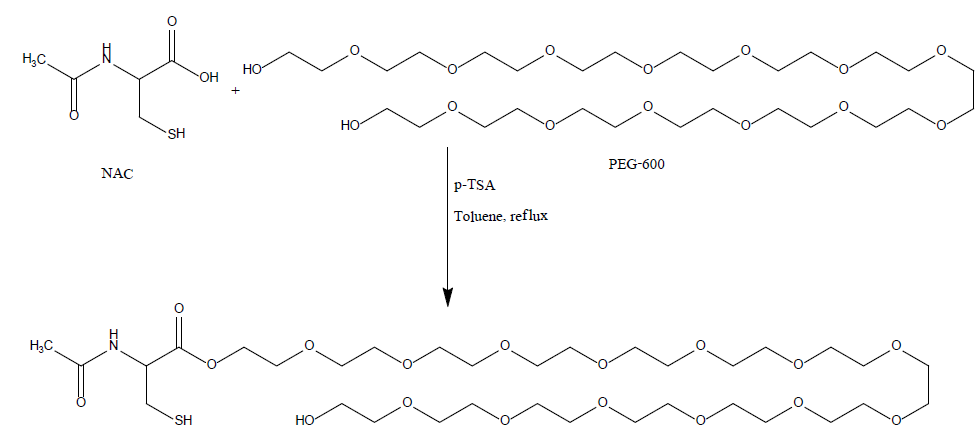
Synthesis of N- acetyl cysteine (NAC) and Polyethylene glycol (PEG) conjugate
Synthetic precursors (N-acetyl-L-cysteine >99%, and p-toluenesulfonic acid monohydrate >98.5%, and Poly (ethylene glycol)-600) were purchased from Sigma-Aldrich without further purification. Solvents (Toluene >99.5%, methyl alcohol "analytical", and dichloromethane "Ar" >99.9%) were purchased from Frutarom and Gadot, respectively, and used without further purification. Deuteriated solvents (DMSO-d6 and D2O) for NMR measurements were purchased from Sigma-Aldrich. Solvents for chromatography were analytical grade. Sodium carbonate (Na2CO3) >99.8% and sodium sulfate (Na2SO4) >99% were purchased from Frutarom. Analytical thin-layer chromatography (TLC) was performed on silica-gel plates with F-254 indicator, visualized by irradiation with UV light. Column chromatography was carried out using silica-gel (Grace) 0.040-0.063 mm (Merck). 1H NMR spectra were recorded on a Bruker Avance 400 (400 MHz, 1H NMR). Chemical shift values (δ) are reported in ppm (TMS δ=0 ppm for 1H; residual DMSO-d6 δ=2.5 ppm for 1H). The proton spectra are reported as follows δ (multiplicity, coupling constant J, number of protons, moiety). Multiplicities are indicated by s (singlet), t (triplet), m (multiplet), and so on.
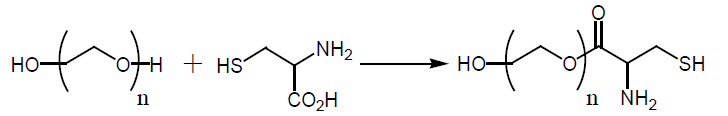
A 250 mL, one-necked, round-bottomed flask equipped with a magnetic stirrer, Dean-Stark trap, and a reflux condenser. The flask was charged with N-acetylcysteine, ("NAC", 7 g, 42.8 mmol) and poly (ethylene glycol)-600, ("PEG-600", 25.8 g, 42.8 mmol)) in toulene (320 mL). P-toluenesulfonic acid monohydrate ("P-TSA", 9.33 g, 49 mmol) was added and the stirred mixture was heated under reflux in an oil bath (about 140°C) for 2-3 hr (the reaction was monitored by TLC). The mixture was allowed to cool to ambient temperature and two phases were observed. The mixture was neutralized by adding sodium carbonate (5 g) and stirred for 2 hrs (gas was bubbled). The solvent was evaporated under reduced pressure and the residue was dissolved in dichloromethane, the precipitate was filtered, the organic phase was dried over dry sodium sulfate, filtered and concentrated with a rotary evaporator to give viscous oil. The resulting residue was purified by column chromatography on silica gel, (as eluent: DCM: MEOH, 95:5) to afford the product NAC-PEG as yellowish oil (22gr, 69%).
Chemical pathway of NAC-PEG-600:
Successful synthesis of small size, NAC modified GNPs
Aqueous dispersion of NAC modified gold nanoparticles was prepared by the described process and imaged using transmission electron microscopy (TEM). TEM images of the sample are shown in Figure 1; mono-dispersed spherical GNPs can be seen while their mean diameter is in the range of 3 ± 0.5 nm. Average diameter of the gold nanoparticles was measured and calculated using imageJ software [18]. As can be seen in Figure 2, the average diameter of the particles is ~ 3.5 nm obtained for different samples.
UV-VIS absorption of gold nanoparticles was measured in spectrophotometer; the spectro-photometry analysis of our samples demonstrated a strong absorption at 510-520 nm as can be seen in Figure 3 confirming the formation of small nano particles. Generally, gold nano particles exhibit a single absorption peak in the visible range between 510-550 nm.
Successful synthesis of PEG-NAC conjugate
1H-NMR analysis of the PEG-NAC conjugate; produced as described above; provided a spectrum that confirms the structure of the product: 1H-NMR (DMSO-d6, 400 MHz) δ: 1.82 (s, 1H, SH), 2.13(s, 3H, CH3), 3.34-3.60 (m, 56H, PEG-CH2O), 4.13-4.22 (m, 2H, CH2S), 5.05 (t, J= 8.1 Hz, 1H, CH), 5.69 (s, 1H, NH).
Synthesis of PEG-NAC modified GNPs
NAC modified GNPs were incubated with PEG-NAC conjugates to allow ligand exchange and formation of PEG-NAC modified GNPs. TEM images of the resulted PEG-NAC modified GNPS are shown in Figure 4. As can be noticed the GNPs kept their original size and shape, however a white corona surrounding them can be observed confirming the linkage of the PEG-NAC to the surface of the GNPs.
Stability test
Both NAC and PEG-NAC conjugates are capable of binding the surface of the GNPs via the thiol group of the NAC, however, we believe that PEG-NAC modified GNPs are much more stable than NAC modified GNPs, to examine that we incubated GNPS modified with NAC only and GNPs modified with PEG-NAC at cell culture medium and monitored their appearance.
NAC modified GNPs demonstrated GNPs- protein interaction; black aggregates were formed at the mixture of cell culture medium and NAC modified GNPs; Figure 5 (Right dish).
However, PEG-NAC modified GNPs incubated at cell culture medium maintained their stability, dispersion and nano size, no formation of aggregates was observed, Figure 5 (Left dish).
Formation of uniform small gold nano particles is still challenging. Furthermore, stabilizing GNPs at protein containing solution is difficult due to the effect of ligand exchange that occurs at the time of mixing GNPs with protein containing solutions. One of the suggested solutions is the use of PEGylation technology which attracts a lot of attention in the field of surface chemistry, due to its unique biocompatibility derived from its own properties such as non-toxicity, solubility and flexibility.
In this work, we developed a new method to synthesis small size and uniform GNPs modified with NAC molecules bond to the surface of the GNPs via a thiol group. Furthermore, we synthesized a novel PEG-NAC conjugate to replace the NAC at GNPs surface for improved stabilization. We observed the behavior of the NAC modified GNPs and the PEG-NAC modified GNPs at protein containing solutions. We noticed that NAC modified GNPs are less stable at protein containing solutions, however, PEGNAC conjugate completely stabilize the GNPs and prevent their interaction with protein; possibly due to the prevention of ligand exchange process. Our approach to conjugate PEG polymer to NAC molecule can replace complicated methods to prepare PEG-SH. Due to their enhanced safety and stability at protein containing solution; GNPs produced by the above method and modified with PEG-NAC molecule have great potential to be used for medical applications such for cancer imaging and radiation enhancement agent. Further work to study their uptake at tumor cells compared to healthy tissue should be designed in the future.
GNPs: Gold nanoparticles; PEG: Polyethylene glycol; NAC: n-acetylcysteine; TEM: transmission electron microscopy
Ayoub A participated in the design of the study and the methods, experiments, and drafted the manuscript. Safadi N participated in the design of the methods, prepared the gold nanoparticles. Inibtawi M performed the UV-VIS measurements, performed the statistical analysis. Shadafny S prepared the PEG-NAC conjugate. All authors read and approved the final manuscript.