ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Pushplata3, Ruchi Gupta3, Gunjan Suri3, Meenu Talwar3, Geetha Seshadri3, M. Sarwar Alam2, R.K. Khandal1*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Hydrogels have gained considerable importance during the last five decades as an industrially useful material. Due to their unique property of water absorption, they find potential applications in soil conditioning by maintain water level of soil, diapers for infants and adults, feminine hygiene products, wound dressings, etc. While synthetic hydrogels are based on water and synthetic monomer (acrylic acid), semi-synthetic hydrogels are based on water synthetic monomer (acrylic acid) and a natural material (gum arabic). In the present study, synthetic (based on acrylic acid) as well as semi-synthetic (based on gum Arabic and acrylic acid) hydrogels have been synthesized using gamma irradiation technique. A comparative study on the gel content and water absorption behaviour of synthetic and semi-synthetic hydrogels has been carried out in the present paper. Through this study, it has been demonstrated that gamma irradiation is an effective tool for the synthesis of hydrogels. The study reveals that stable and cross-linked hydrogels have been obtained after subjecting to ï§- irradiation. Water absorption of semi-synthetic hydrogels was found to be higher than that of synthetic hydrogels.
Keywords |
| Gamma irradiation, gum arabic, acrylic acid, hydrogels, gel content, water absorption and metal ions. |
INTRODUCTION |
| Hydrogels have emerged as a novel material and have led to great advancement in the field of pharmaceuticals and biomedical products. Hydrogels are cross-linked, three-dimensional polymer networks having hydrophilic functionalities. They are capable of absorbing large amounts of water, saline and other biological fluids [1-3]. In other words, hydrophilic polymers get crosslinked, chemically or physically to form three- dimensional networks which makes the material swell in water or any other biological fluid while remaining insoluble [4]. The hydrophilic nature of hydrogels is due to the presence of polar groups such as –COOH, –OH, –HSO3, –CONH2, etc. on the polymer chain. The structure of hydrogel has been shown in Figure 1. |
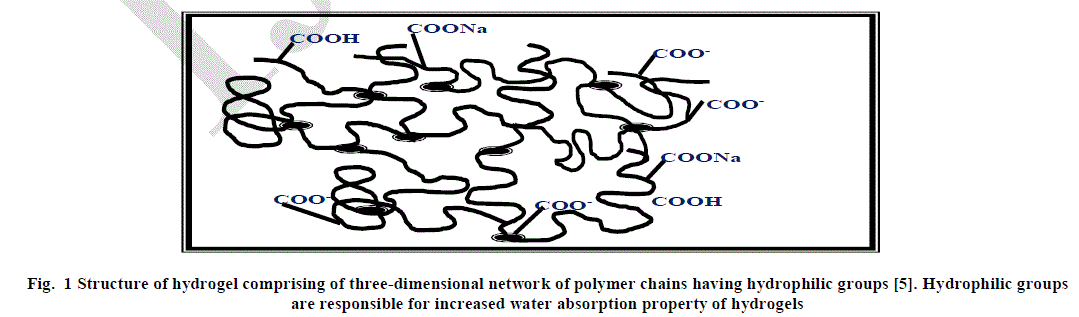 |
| Recently, hydrogels have found a wide range of medical applications like drug delivery, wound dressing, contact lenses, etc. The success of hydrogels as compatible biomaterials lies in their resemblance to living tissues because of their high water content which minimizes the friction around the surrounding tissue. The relatively high water content of hydrogels has also made them permeable to oxygen, nutrients and metabolites, making them ideal materials of choice as devices for the controlled release of drugs and other active agents, as industrial absorbent and in personal hygiene products . |
| Hydrogels may be based on synthetic or natural polymers. In case of synthetic hydrogels, monomers such as acrylic acid (AA), hydroxyethylmethacrylate (HEMA), N-(2-hydroxy propyl) methacrylate (HPMA), N-isopropylacrylamide (NIPAMM), N-vinyl-2-pyrrolidone (NVP), methacrylic acid (MAA), vinyl acetate, acrylamide, etc have been used so far for their synthesis because these materials possess excellent water absorbing properties[6]. Despite several advantages of these hydrogels, there are certain major limitations associated with their use. Their inherent toxicity and non- biodegradability are the two major shortcomings, which limits their use in drug delivery systems. Because of these shortcomings, natural polymer based hydrogels are preferred [7]. A number of ionic hydrogels based on chitosan, alginate and hyaluronic acid have been explored for their use as scaffolding materials for bone cartilage, cornea, heart valve, etc [8-10]. |
| Natural polymer based hydrogels are usually prepared by reacting polysaccharides with a synthetic monomer in the presence of an initiator at certain temperatures [11]. The reaction results in the formation of a hydrogel comprising of a cross-linked network of monomer and natural polymer. |
| In the present study, gum arabic (polysaccharide) and acrylic acid (synthetic monomer) based hydrogels have been synthesized. Gum arabic (GA) is a plant hydrocolloid and is known to be a potent antioxidant [12-13]. Its key features are high solubility, pH stability, non-toxicity and gelling characteristics. Gum arabic is a highly branched, complex acidic hetero-polysaccharide with main chain of (1ïÃâî3)-ß-D-galactopyranosyl units and side chains containing Larabinofuranosyl, L-rhamnopyranosyl, D-galactopyranosyl, and D-glucopyranosyl uronic acid units. It is extensively used in a wide range of applications such as confectionery, beverage or liquid flavor emulsions, pharmaceuticals, cosmetic products, inks, etc. Acrylic acid (AA) is a hydrophilic pH responsive monomer and has been extensively used in the preparation of hydrogels. |
| A novel methodology such as gamma irradiation has been used for the synthesis of hydrogels. The major advantage of this technique is that it is a cold process and does not require the use of any initiators, which are known for their high cost and toxic nature. |
| The aim of this study is to replace a part of the synthetic monomer (acrylic acid) by the natural polysaccharide (gum arabic) and replace the conventional method of hydrogel synthesis i.e. thermal polymerization by radiation polymerization technique. |
EXPERIMENTAL |
A. Materials |
| The materials used for the preparation of hydrogels were procured from local sources. Acrylic acid (AA), Calcium Chloride (CaCl2), Ferric chloride (FeCl3) and Gum arabic (GA) were procured from sd Fine Chemicals Limited, Mumbai (India) and Sodium chloride (NaCl) was procured from Hi-Pure Rankem. Limited, Gujarat (India). These materials were used as available without any further purification. |
B. Methodology for the Preparation of Hydrogels |
| Hydrogels were prepared by carrying out the polymerization of water - acrylic acid and water-acrylic acid-gum arabic mixtures by gamma irradiation at different doses from 5 to 200kGy at atmospheric conditions at a dose rate of 25kGy/ 12hrs Figure 2. Co-60 was used as the source of ïÃÂç-radiation. The hydrogels obtained were characterized for the properties listed under characterization. |
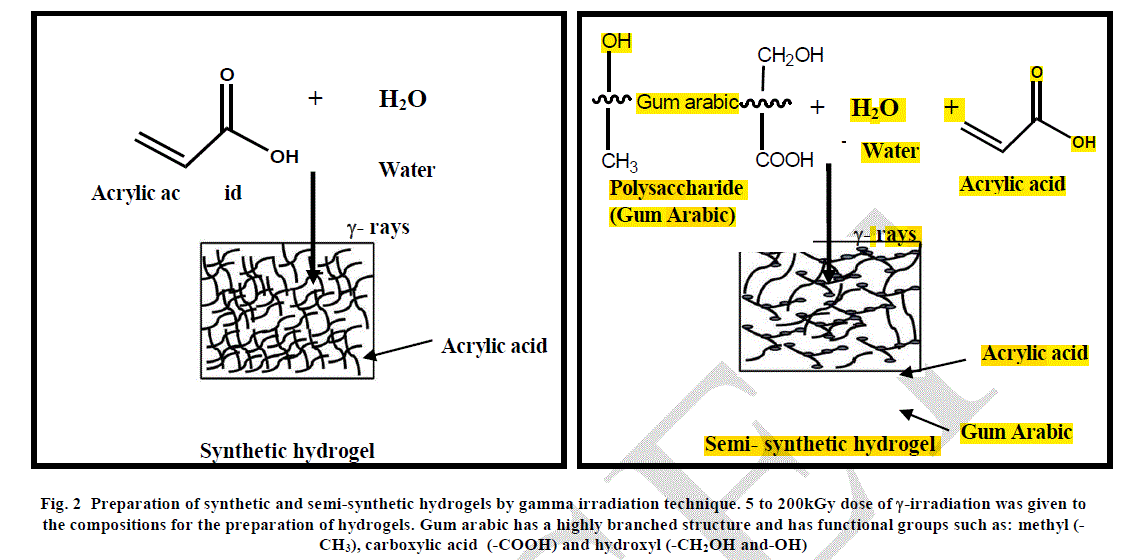 |
C. Characterization |
| The hydrogels synthesized in this study were characterized for gel content to determine the extent of cross-linking and water absorption behaviour to establish their structural attributes. |
(i) Gel content |
| In order to determine the gel content, a known weight of the hydrogel was subjected to Soxhlet extraction in water for 24 hrs. After extraction, the hydrogel was dried in an oven maintained at 100 ± 5°C till constant weight was obtained. The gel content was calculated using the following formula: [14] |
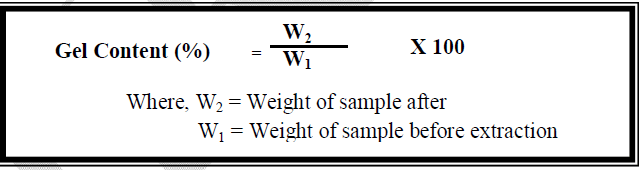 |
(iii) Viscosity |
| The viscosity studies have been carried out by using Cannon fenske viscometer (capillary size 0.0168) and Brookfield viscometer (model LVDV-II +P) at 25ïÃâðC. In this study, the mixture of water and acrylic acid were prepared by varying the water: acrylic acid ratio to study the solubility of gum arabic in these mixtures. Gum arabic was slowly added under stirring till homogeneity of solution was obtained. |
III. RESULTS AND DISCUSSION |
A. Solubility of gum arabic in water-acrylic acid mixture: |
| The solubility of gum arabic was evaluated in water-acrylic acid mixture of different ratios from 0-100 % to study the solubility of gum arabic in these mixtures. Gum arabic was slowly added under stirring till homogeneity of the solution was obtained. 0 to 40 % gum arabic was obtained to check its extent of solubility in water-acrylic acid mixture and it was observed that a minimum water content of 70% is required in the mixture to enable the solubilization of gum arabic. In order to achieve the maximum replacement of acrylic acid with gum arabic, water: acrylic acid (70:30) mixture was selected for the synthesis of hydrogels used in this study. |
B. Viscosity of gum arabic-water-acrylic acid mixture: |
| The effect of addition of gum arabic on viscosity of water acrylic acid mixture of 70:30, 80:20 and 90:10 ratios has been studied to find out the maximum amount of gum arabic that can be incorporated in water-acrylic acid mixture. The results have been shown graphically in Figure 3. As evident from the figure, upto 40% of gum arabic could be added in all three mixtures. In case of water: acrylic acid ratio of 70:30, maximum viscosity of 2888 cps could be achieved at 40% gum arabic concentration. Beyond 40%, paste formation took place and flowability of the gum arabicwater- acrylic acid mixture was lost. Keeping this in view, 40% gum arabic concentration in 70:30 water: acrylic mixture was selected for the present study. Another point worth mentioning here is that in the case of all the three mixtures of water and acrylic acid, a steep rise in viscosity was observed at more than 30% concentration of gum arabic in these mixtures. This indicates that when the concentration of gum arabic exceeds 30 % in the mixture, the solutesolute interactions become predominant. In other words, at concentration more than 30%, the interactions are long range whereas at concentration more than 30%, the interactions are short range. |
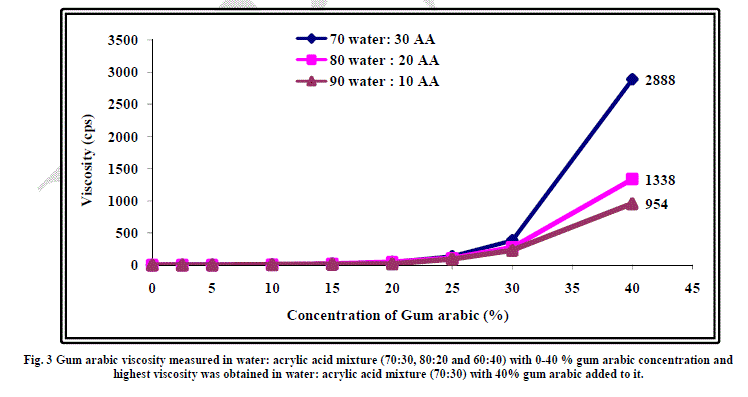 |
C. Effect of irradiation on gel content of hydrogels: |
| When water and acrylic acid are mixed, they form a homogeneous solution which can be attributed to the hydrogen bonding interaction between the two leading to hydration of acrylic acid molecules. When this mixture is irradiated free radicals are generated, polymerization takes place due to the unsaturation present in acrylic acid. The water present in this mixture gets entrapped in the cross-linked network leading to hydrogel formation. |
| When gum arabic is added to water-acrylic acid mixture, hydration take place by hydrogen bonding interaction with water and acrylic acid. Gum arabic has a highly branched structure and has functional groups such as: methyl (-CH3), carboxylic acid (-COOH) and hydroxyl (-CH2OH and -OH) which is copolymerized with acrylic acid leading to the formation of highly crosslinked network. This is the reason behind higher gel content of this semi-synthetic hydrogel at 5kGy radiation dose as compared to the gel content of synthetic hydrogel at the same dose. On increasing the radiation dose, gel content was found to increase from 69.2% at 5kGy to 74.9% at 200kGy in case of the synthetic hydrogel. This shows that cross-linking increases on increasing the radiation dose but at the same time cross-linking is not very high, the maximum being about 75%. This can be attributed to the presence of physical hydrogen bonding interaction between water and acrylic acid which keeps these molecules at a distance leading to lower gel content. |
| In case of the semi-synthetic hydrogel, the highly branched structure of gum arabic acts as a bridge between acrylic acid molecules leading to a higher gel content of 80% at low radiation dose of 5kGy. As the radiation dose is increased further, depolymerization of gum arabic takes place, which leads to reduction in the gel content as shown in Figure 4. |
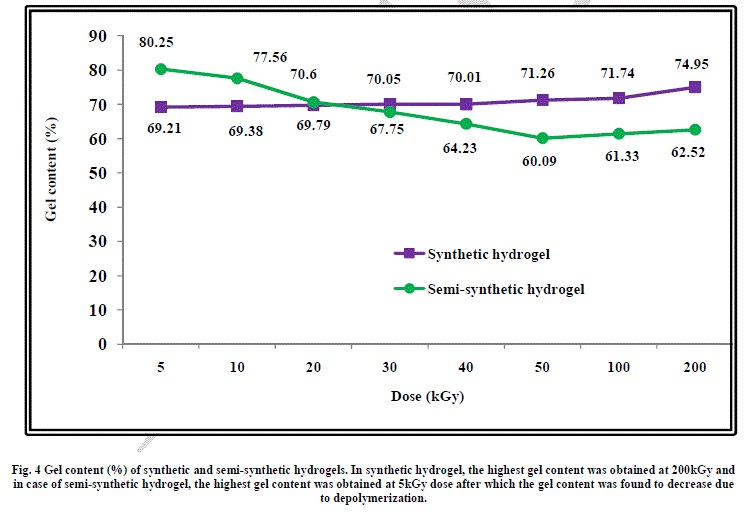 |
D. Water Absorption |
I. Water absorption behaviour of synthetic hydrogels |
| The effect of radiation dose on water absorption of hydrogel has been shown in Fig. 5. As evident from the graph, water absorption increases with time and decreases with radiation dose. |
| As discussed earlier, the gel content or the cross-linking increases with the increase in irradiation dose which means that the hydrogel gets more compact on increasing the radiation dose. The water penetration gets reduced as the hydrogel becomes more compact, thereby reducing the water absorption. This is evident from the graph shown in Figure 5. The maximum water absorption is exhibited by the hydrogel formed by irradiating water-acrylic mixture at 5kGy dose. |
II. Water absorption behaviour of semi-synthetic hydrogels |
| The water absorption pattern exhibited by the semi-synthetic hydrogel with the increase in irradiation dose has been shown in Figure 5. |
| As described earlier, the semi-synthetic hydrogel formed by irradiation at various doses has a loose or fluffy structure with lots of spaces in it. This has made the absorption of water easier giving rise to higher water absorption in case of semi-synthetic hydrogel than in synthetic hydrogel. |
| As the radiation dose was increased, water absorption was found to increase. This is because the space in the crosslinked structure of the hydrogel increased leading to better absorption ability. Similar trend was observed in the case of water absorption in different hydrogels. |
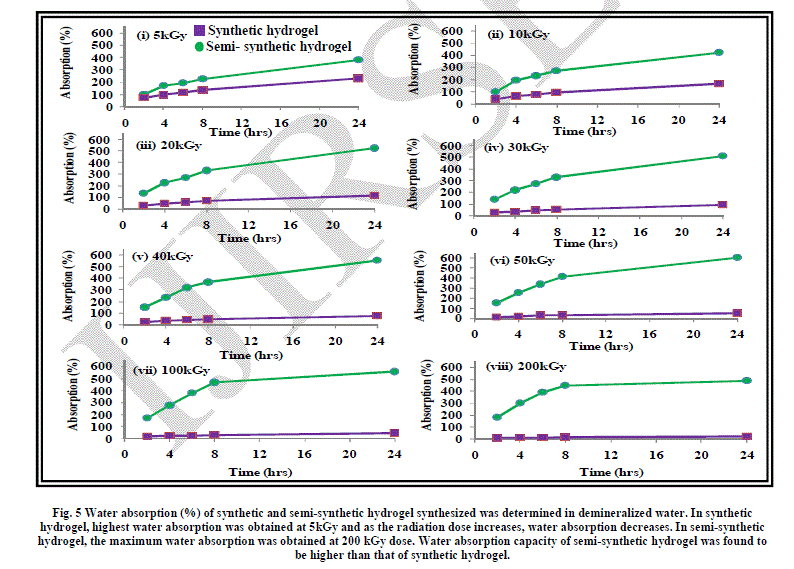 |
II. Water absorption behaviour of synthetic hydrogels in the presence of ions |
| The effect of addition of salts of mono, di and trivalent cations has been studied on the absorption behaviour of synthetic hydrogels Figure 6, 7 and 8. As evident from the results, absorption of synthetic hydrogels was found to increase with increase in pH of solution. Also, absorption was found to decrease with the increase in radiation dose. This can be attributed to the cross-linking of the acrylic acid molecules taking place with increase in radiation dose. The hydrogel is compact and becomes more compact on increasing the radiation dose. The hydrated metal ions present in water have large diameters and are not able to enter into the space in the structure of hydrogel and thus the absorption is low. In case of 1% FeCl3 aqueous solution, no absorption by synthetic hydrogel was evident. |
IV. Water absorption behaviour of semi-synthetic hydrogels in the presence of ions |
| The trend of water absorption by semi-synthetic hydrogels in presence of various salts has also been shown in Figure 6, 7 and 8. |
| As evident from the graphs, the water absorption was found to be lesser in case of salt solutions as compared to that in water. This can be attributed to the fact that the osmotic pressure difference between the hydrogel and the salt solution is less as compared to that between hydrogel and water, which leads to reduction in absorption by the hydrogel. Another aspect responsible for lesser absorption can be that in the case of salt solutions, water along with the cations have to be absorbed by the hydrogel. The spaces in the cross-linked network of hydrogel can take up only a limited amount of cations. An evidence of this is apparent when we see that with the increase in the size of the cations: Fe3+ > Ca2+ > Na+, the absorption decreases. |
| It is imperative to mention here that the metal ions form hydrated complexes or metal aqua ions in aqueous medium, which have varying pKa values. The pKa values are dependent on size, charge and electronegativity. Lower is pKa, higher is the acidity of the metal aqua ion. The pKa value of Na+ is the highest at 14.1 among the three cations being studied, leading to lowest acidity of its metal aqua ion. Fe3+ has the lowest pKa value of 2.2, which make it a strong acid. The hydrogel is acidic in nature due to the presence of carboxylic acid groups and Fe3+ solution is also acidic. This also retards the movement of water /ions from Fe3+ salt solution into the hydrogel and thus, the absorption is least in the case of 1% FeCl3 solution. |
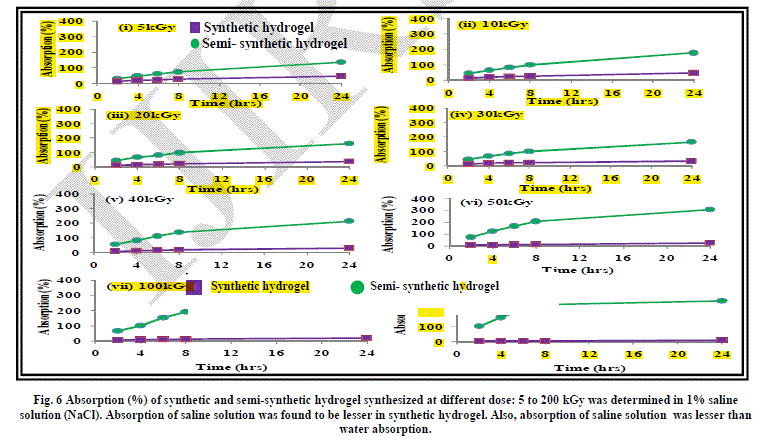 |
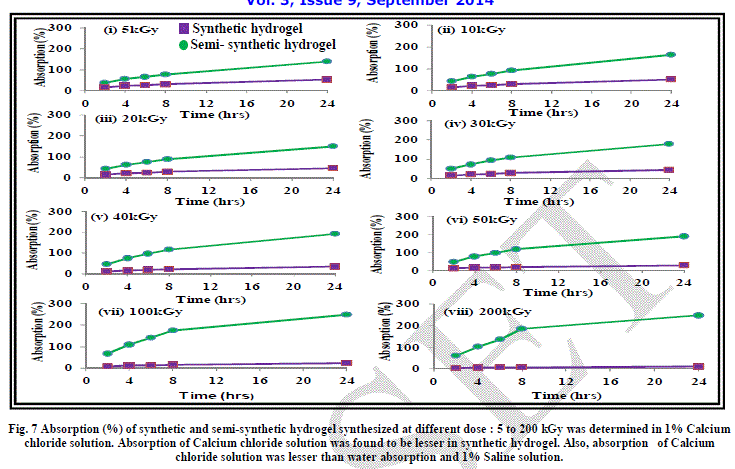 |
IV. CONCLUSION |
| Through this study it has been demonstrated that hydrogel synthesis can be carried out by using gamma irradiation technique. Semi-synthetic hydrogels are found to exhibit better property than synthetic hydrogels. Synthetic hydrogels were synthesized using water-acrylic acid mixture with a maximum gel content of 75% at 200kGy of gamma radiation. Semi-synthetic hydrogels were synthesized using gum arabic with acrylic acid, where a maximum gel content of 80% was achieved at 5kGy radiation. Water absorption in the case of semi-synthetic hydrogel was found to be higher than that of synthetic hydrogel with maximum water absorption of 600% compare to 230% in the case of synthetic hydrogels. The trend of water absorption in the presence of metal cations was found to be in the order of Na+>Ca 2+> Fe3+. The novel idea of preparation of semi-synthetic hydrogels using gamma irradiation technique presented in this study has the paved the way for adoption of this technique industrially. This has a lot of potential applications in many medical areas. The semi-synthetic hydrogel discussed in the present study can be explored for its application in the areas where synthetic hydrogels are being are used: hygiene products, soil conditioners, water purification etc. |
V. ACKNOWLEDGEMENT |
| The authors express sincere gratitude to the Management of Shriram Institute for Industrial Research, Delhi, India, for the kind support. |
References |
|