ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S.Sharmila1, B.Janarthanan2, B.Gomathi1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Cubic spinel shaped Li4Mn5-xZnxO12 (x= 0.0, 0.25, 0.5 and 0.75) has been synthesized by single step molten salt method at 800oC for 10hr. Sample has been characterized by XRD, SEM, impedance and conductivity analysis at different temperature. Results have shown good conductivity for 0.1 mole Zn doped Li4Mn5O12
Keywords |
| Li-ion battery, molten salt method, conductivity and impedance analysis. |
INTRODUCTION |
| Batteries play a vital role to fulfill the requirements of storing electrical energy. At present Li-ion batteries are available commercially, graphene and Cobalt based Li electrodes are used as anode and cathode material for rechargeable Li-ion batteries. An alternate material is indispensable for cobalt as the capitalization cost is very high and toxic [1]. In this scenario, Manganese based compounds (LiMn2O4, LiMn2O3 and Li4Mn5O12) has shown an immense satisfaction as an alternate to cobalt due to its harmless nature, low cost, wide operating potential window, long shelf life and high cell voltage [2]. Thackery et al., [3] have reported the merits of LiMn2O4 accompanied with changes in symmetry and also Jahn – Teller distortion due to its reduction in oxidation state of Mn4+ during cycling, which can be reduced by aliovalent substitution in Mn sites [1]. Also the Increase in oxidation state leads to good cycling stability in 3V region. |
II. BACKGROUND and RELATED WORK |
| Choi et al., [4] has made an attempt by anionic substitution of F- in O2- sites which suppress the disproportionate of Li4Mn5O12 and also exhibits good capacity. Further Yan jing Hao et al., [5] have confirmed the Li4Mn5O12 electrode as a symmetric supercapacitor with good capacity behavior. Jiang et al., [6] has synthesized Li4Mn5O12 nano-crystallites by the combined action of low temperature spray-drying with solid phase reaction for first time. Li4Mn5O12 has been synthesized by employing high energy ball milling technique by Julien et al., [7] and exhibited the capacity of 158 mAh/g. Moreover by using molten salt method Li4Mn5O12 nanowires have been synthesized by Yang Tian et al., [8] with good charge/discharge curves between 2.3 and 3.3 V. Followed by the researches, researchers (Yan Zhao et al., [9], Yan-jang Hao et al., [10], Robertson et al., [11]) have carried out extensive work on Li4Mn5O12. In the present work, an attempt has been made to prepare Zn doped Li4Mn5O12 in order to increase the conductivity by molten salt method. |
III. MATERIALS AND METHOD |
| Li4Mn5-xZnxO12 (x=0.0, 0.25, 0.5 and 0.75) has been prepared by molten salt method using LiOH.H2O, MnO2 and ZnO as starting precursors with LiCl - KCl as flux. The stoichiometric quantities of starting compounds are grounded and mixed well with flux. Obtained slurry has been calcined at 800ºC for 10 h and then allowed to cool to room temperature. The resultant products have been washed for several times with distilled water and then with ethanol to remove the residual molten salts and then dried. Powder XRD pattern using CuKα radiation has been used to identify the phase structure and to calculate the lattice parameter. The investigation of the morphology of the prepared samples has been examined by scanning electron microscope and Electrical conductivity of the prepared materials by using HIOKI 3532 LCR HI-TESTER in the frequency range of 42Hz – 1MHz at various temperatures. |
IV. RESULT AND DISCUSSION |
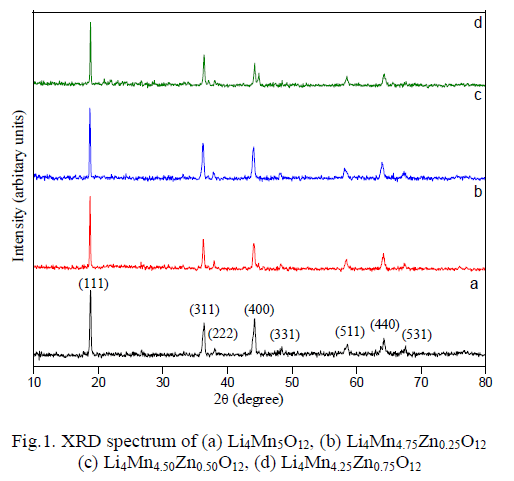
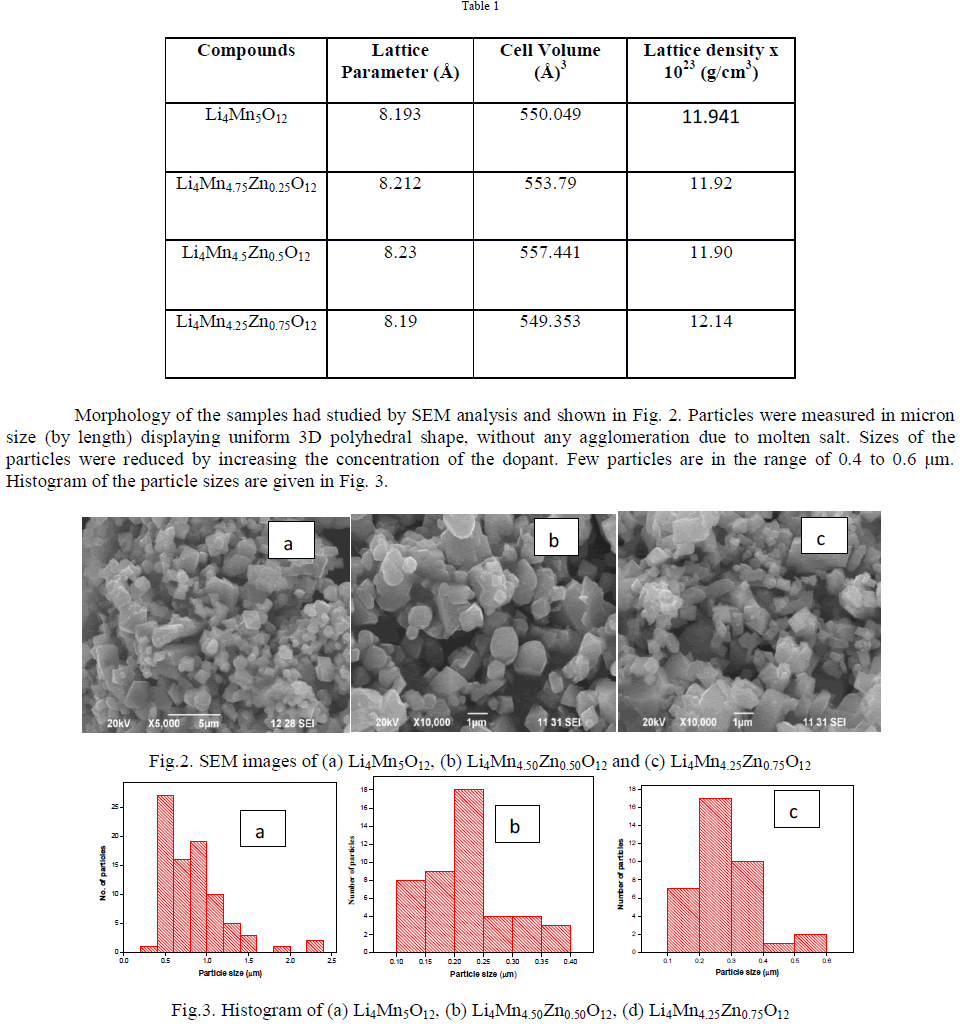
| XRD pattern has carried out to confirm the nature of the material, which is shown in Fig. 1a. It was observed that the formation of highly crystalline nature of the material without any impurity. All the diffraction peaks are matched well with the standard JCPDS card no. 46-0810 indicating the formation of pure Li4Mn5O12. Observed peaks were indexed to (111), (311), (222), (400), (331), (511), (440) and (531) plane in the form of spinel cubic structure with Fd3m space group. XRD spectrums of doped samples are shown in Fig. 1(b-d), whose peaks also matched well with parent material, confirms that Zn entered into Mn lattice without any changes in its structure. Lattice parameter of all the samples were calculated and given in Table 1. Lattice constant of doped materials are found to be higher than pure material due to the difference in ionic radii of Mn4+ (0.535Å) and Zn4+ (0.74 Å) which follows vegard’s law [12]. Similarly, due to the difference in atomic density of Mn4+ and Zn4+ the lattice density of doped materials were found to be higher than pure Li4Mn5O12 [12]. |
 |
 |
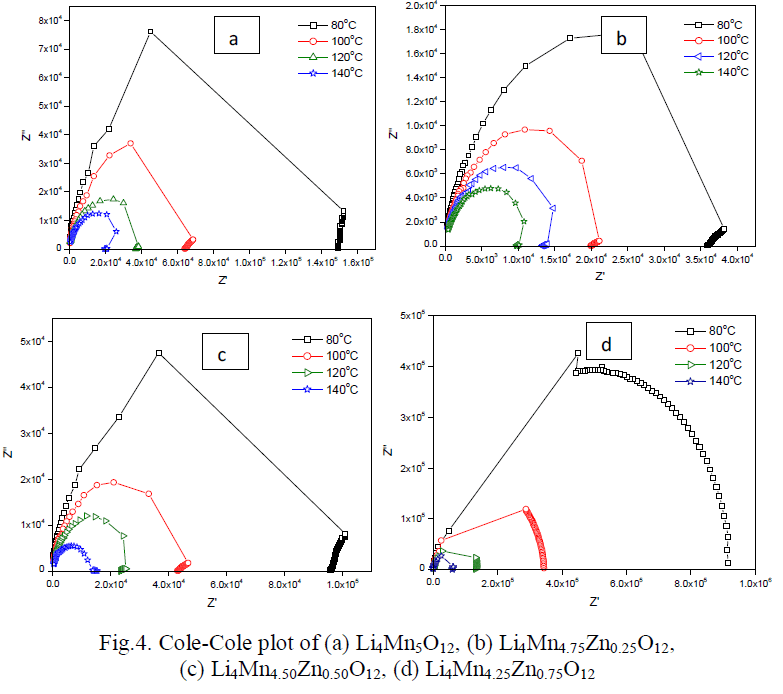
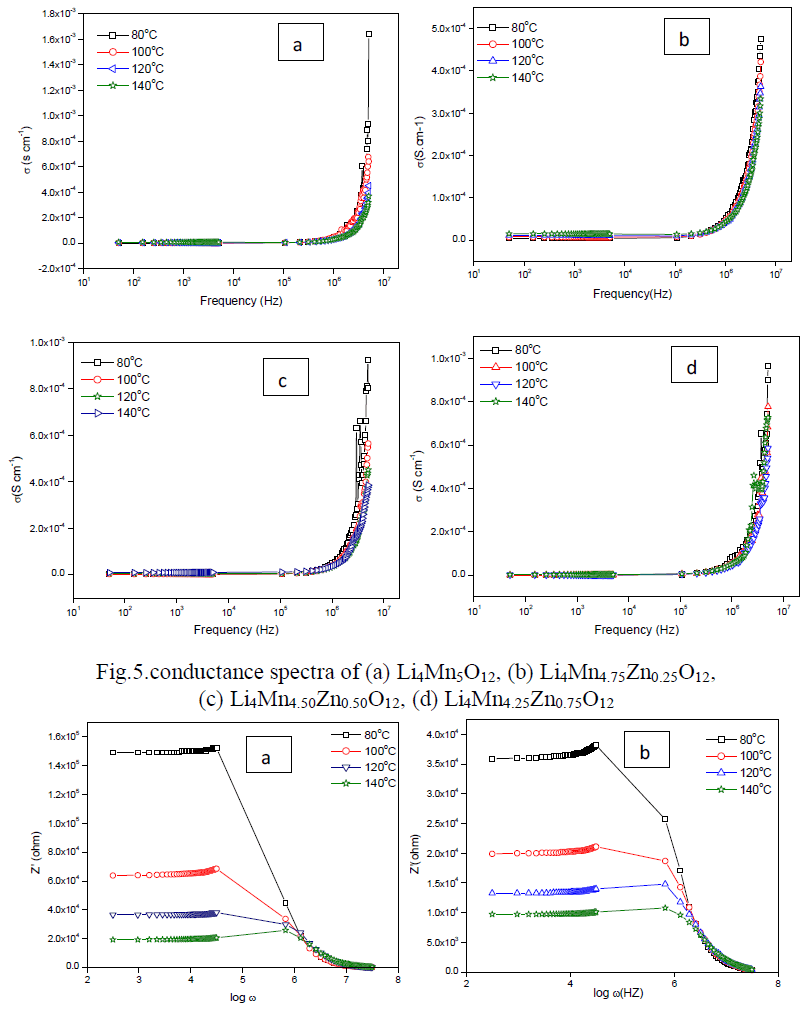
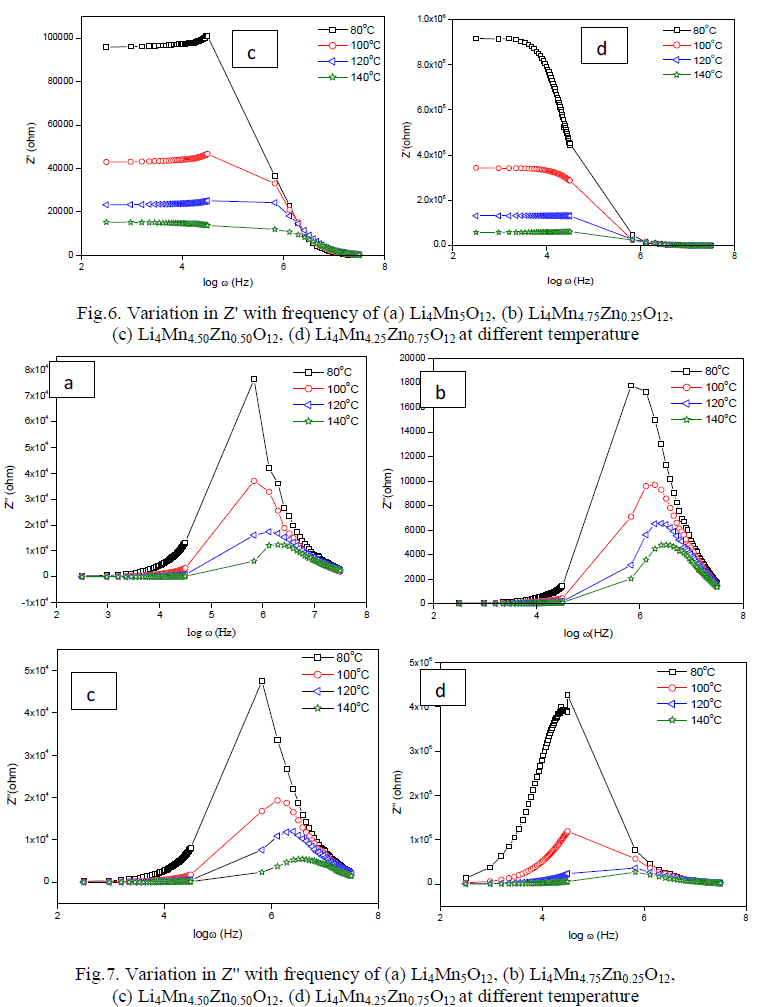
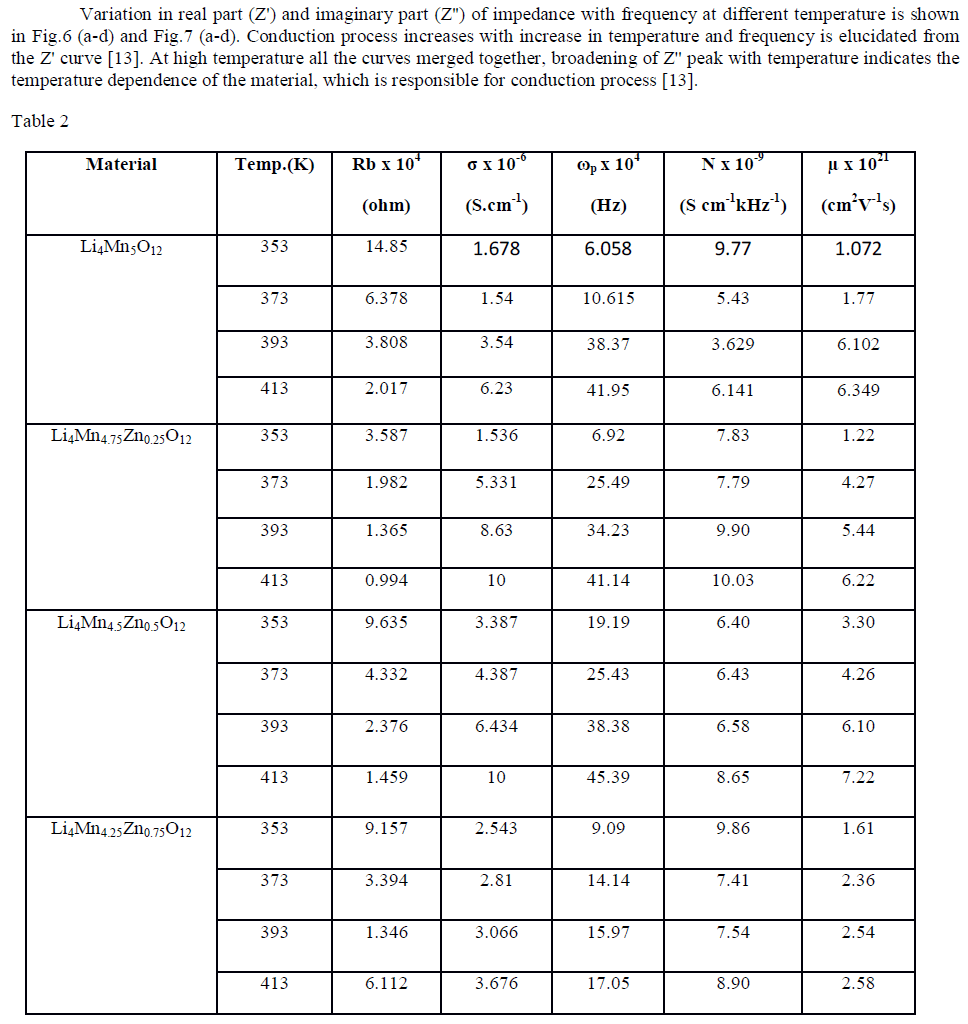
| Cole-Cole plot of Li4Mn5-xZnxO12 were studied by electrical properties at four different temperatures such as 353 K to 413 K. Fig.4(a-d) indicates the formation one semi-circle due to the parallel combination of R-C reveals that the conduction process occurs through bulk of the material i.e. grain interior [13]. Bulk resistance (Rb) of the material can be obtained by intercepting the semi-circle on the x-axis. Rb values decreases with increase in temperature i.e. negative temperature coefficient of resistance (NTCR) property, indicates the semi-conducting nature of the material [13]. The center of the circle falls below the real axis elucidates the non-Debye nature of the material. Conductance spectra of all the samples are shown in Fig.5 (a-d) dc conductivity of all the samples were calculated and given in Table 2. Compared to pure material Zn doped sample shows high conductivity except Li4Mn4.25Zn0.75O12. |
 |
 |
 |
 |
V. CONCLUSION |
| The following conclusions have been drawn on the present work and are 1. Zn doped Li4Mn5O12 synthesized at single step molten salt method and Cole-Cole plot inferred that the conduction process is due to the bulk of the material and NTCR property of the material. 2. Li4Mn4.75Zn0.25O12 exhibited enhanced conductivity than other materials (Li4Mn5O12, Li4Mn4.5Zn0.5O12 and Li4Mn4.25Zn0.75O12 ). 3. The optimum concentration of the dopant is found to be 0.25 mole which has significant impact on the efficiency of the material. 4. It can be confirmed that Li4Mn4.9Zn0.1O12 is a better alternate material for LiCoO2. |
ACKNOWLEDGEMENT |
| The authors would like to thank Dr.J.Chandrasekaran, Associate Professor, Department of Physics, Sri Ramakrishna Mission Vidyalaya College of arts and Science, who has provided the facility to complete the work. |
References |
|