e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Sumantha Malur Gopalakrishna1, Girisha Sirangala Thimappa1*, Ramesh Puttalingaiah Thylur2, Yogisha Shivanna2, and Anand Sreenivasan2.
1Dept of Microbiology and Biotechnology, Bangalore University, Bangalore 560056, Karnataka, India.
Received: 09/09/2014; Revised: 22/09/2014; Accepted: 27/09/2014
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The anticancer activities of Solanumindicum, Rhus succedaneum, Rheum emodi, and Gardenia gummifera medicinal plants from the methanolic extracts were determined by using in-vitroMTT cytotoxicity assay in various cancer cell lines human non-small cell lung carcinoma(H1975), prostatecarcinoma (PC-3and DU145), colorectal carcinoma (HCT116) and malignant melanoma (A375). The in-vitrocytotoxicity assay revealed that S. indicum, R. succedanea, R. emodi and G. gummifera extracts had strongest cytotoxicity with IC50 of 8.48 ∞g/ml,24.5∞g/ml, 28.24∞g/ml and 28.61∞g/ml respectively in DU145 cells and also with IC50 of 11.18∞g/ml, 11.04 ∞g/ml, 11.05 ∞g/ml and 23.03∞g/ml respectively in PC-3 cells. Whereas these extracts exhibited cytotoxicity with IC50 of 9.03∞g/ml, 7.71∞g/ml, 15.95 ∞g/ml and 11.71∞g/ml respectively in H1975 cells. Similarly all these extracts showed cytotoxicity with IC50 of 17.58∞g/ml,8.87∞g/ml,19.31∞g/ml and 11.67 ∞g/ml respectively in HCT116 cells. Finally methanolic extracts of these extracts exhibited cytotoxicity with IC5027.94 ∞g/ml, 13.13∞g/ml, 11.05 ∞g/ml and 7.816∞g/ml respectively in A375 cells. Our results revealed that methanolic extract of S. indicum, R. succedanea, R. emodi, and G. gummifera had profound cytotoxic effectand further studies to be initiated for potential herbal formulations to be used as an alternative of chemotherapeutic drugs due to less toxic to normal cells.
Cancer, Cytotoxicity, Colorectal, Lung, Prostate.
Cancer is a multi-step disease developed by physical, environmental, metabolic, chemical and genetic factors, which play a direct and/or indirect role in the induction of cancers [1]. About 10 million new cases are diagnosed and over 6 million deaths occur worldwide annually because of cancer [2]. The risk of developing cancer generally increases with age [3]. The chances of surviving the disease vary greatly by the type, location of the cancer and the extent of disease at the start of treatment. Chemotherapy with plant-derived phytochemicals has appeared as an accessible and promising approach to cancer control and management [4].
Several plants have been shown to be sources of therapeutically important agents, beneficial in the cancer therapy. For example, many cancer chemotherapeutic drugs including vinca alkaloids vinblastine and vincristine isolated from Madagascar periwinkle [3,5], (paclitaxel(Taxol) and docetaxol from Taxusbrevifolia Nutt [6], podophyllotoxin from roots of podophyllum [7], camptothecin from bark of camptothecaacuminate [8] and many more.
The study shows Solanumindicum (Solanaceae) is found throughout south Asia, commonly known as “Begun” in India. The different parts (fruits, leaves, roots) of this plant were traditionally used in the treatment of loss of appetite and anorexia, blood disorders, rhinitis, cough, asthma, sore throat and hiccup, sexual disorders, abdominal pain and worm infestation, pain and fever, in-flammation, insomnia, urinary complications, cardiac weakness etc. The fruits, leaves and root of this herb contain wax, fatty acid, alkaloids etc. Dios-genin, lanosterol, sitosterol, solasonnine, solamargine, solanine, solanidine and solasidine already isolated from this plant [9]. Also ethanobotanical study reveals its efficacy against hypersensitivity, diabetes and jaundice [10,11,12]. Rhus succedanea L. (Anacardiaceae) also found throughout Asia. In Indian ethno medicine, this plant is locally known as Kakrasingi and has been used as ayurvedic remedy for diarrhoea and dysentery [13]. It has been reported to possess antiviral [14], antibacterial [15] and cytotoxic properties [16]. Rheumemodi is commonly known as Himalayan rhubarb, is a medicinal herb used in the Indian Ayurvedic system of medicine usually found in India. Anthraquinones are the major constituents of R. emodirhizomes. It is used to treat kidney stones and other liver associated disease like gout and jaundice [17,18]. It has been proved that the extracts of R. emodi possess an antimicrobial activity against Helicobacter pyroli [19]. Gardenia gummifera belongs to family Rubiaceae is a flowering shrub known by different names in India. The gum resin of this plant is called Dikamali [20]. This gum possesses number of medicinal properties which include anthelmentic antispasmodic, anti-epilepticcardiotonic and antioxidant potential [21,22]. Cycloartanes isolated from the Gardenia gummiferais found to possess cytotoxic and anti-HIV activities [23,24,25,26,27].
A survey of the literature revealed that no studies on the anticancer potential of extracts from these plants have been undertaken on five human cancer cell lines associated with four different cancer: human non small cell lung cancer (H1975), prostate cancer cell line (PC-3 and DU-145), colorectal cancer cell line (HCT116) and skin malignant melanoma (A375). It is found that the same anticancer compound shows variation in the effects for different cell lines. Thus, the use of different cell lines becomes more valuable for the evaluation of anticancer effects.
In the present study we undertook the task to investigate the anticancer activity of crude methanolicextracts of Solanumindicum(Fruit), Rhus succedanea (Stem), Rheum emodi (resin), and Gardeniagummifera (resin/stick)in five human cancer cell lines of different histological origin. Interestingly all the extracts showed dose dependent manner in anticancer cells and hemolytic assay revealed that these extract had no effect on erythrocytes. The findings of the study will be significance for the development of new anticancer therapeutic agents.
Plants materials of Solanumindicum (Fruit), Rhus succedanea a(Stem),Rheum emodi(Resin) andGardenia gummifera (Resin/sticks) were collected from the AmrutaKesari Depot, Bangalore.
The air-dried and powdered plant materials (20g of each) were extracted with 250 ml methanol (CH3OH) by Soxhlet apparatus 60°C, 8hrs [28,29]. The crude extracts were filtered and the filtrate evaporated using a rotary evaporator. The dissolved constituents were further dried under pressurized vacuum conditions. Stock solutions were prepared by dissolving the dried residue in Dimethylsulphoxide (DMSO). Extract solutions were stored at −20°C until use.
In this study we have used six different cancer cell lines were derived from human non-small cell lung carcinoma(H1975), prostate carcinoma (PC-3 and DU145), colorectal carcinoma (HCT116)andmalignant melanoma (A375)were obtained from the American Tissue Culture Collection (ATCC). H1975, PC-3, DU145 and HCT116 cells were grown in Roswell Park Memorial Institute medium (RPMI-1640)and A375 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were incubated at 37°Cin a 5% CO2 humidified incubator.
The cytotoxic assay detects the reduction of MTT [3-(4, 5-dimethylthiazolyl)-2, 5-diphenyl-tetrazolium bromide] by mitochondrial dehydrogenase to blue insoluble formazan product, which reflects the normal functioning of mitochondria and hence the cell viability. Briefly 5.0 X 104 cells of DU145, PC-3, H1975, HCT116 and A375 were plated in triplicate in 96 well plates with RPMI-1640 or DMEM and incubated for 24 hrs at 37°C. Plant extracts were tested as 0, 2, 4, 8, 16, 32, 64 and 128 μg/ml in serum free RPMI media and incubated for 24 hr in CO2 incubator at 37°C. After incubation with plant extracts, the media was removed from the wells and added 100 μl/well of the MTT reagent and incubated for 3-4 hrs. After incubation, the MTT reagent was removed before adding 100 μl DMSO to each well and gently shaken. Plant extracts treated cells were compared to untreated cell control wells. Measure the absorbance at 590nm using a Tecanmicroplate reader. The percentage inhibition was determined using the formula.
% Inhibition = 100-(optical density of sample/optical density of control) × 100.
IC50 values were calculated as the concentrations that show 50% inhibition of proliferation on any tested cell [30,31,32,33].
Five ml of blood was collected from healthy volunteers in the tubes containing 5.4 mg of EDTA to prevent coagulation and centrifuged at 1000 rpm for 10 min at 40C. Plasma was removed carefully and the white buffy layer was completely removed by aspiration with a pipette with utmost care. The erythrocyteswere then washed for additional three times with 1X PBS, pH 7.4. Washed erythrocytes were stored at 47deg;C and used within 6 h for the haemolysis assay [34,35,36].
Haemolytic activity is a method to study the interactions between blood and biomaterials may induce erythrocytelysis. In this study we have explained briefly take 50μl of 10 dilution (100μl Erythrocytes suspension: 900μl 1X PBS) of erythrocytes suspension into 2ml of new eppendorf tube and incubated with 100μl of various concentration of plant extracts (0, 2, 4, 8, 16, 32, 64 and 128 μg/ml) at 370C water bath for 60-90 min. Here we were using 100μl of 1X PBS as negative control and 100μl of 1% SDS as positive controls and then adjusted the volume of reaction mixture to 1ml by adding 850μl of 1X PBS. Finally centrifuged at 300 rpm for 3-5 min and the resulting hemoglobin insupernatant were measured at 540nm by Tecan micro plate reader. Data Analysis Software to determine the concentration of haemoglobin [34,35]. The haemolysis caused by 100 μl of 1% SDS was taken as 100% haemolysis; and the percentage of haemolysis was calculated by the equation
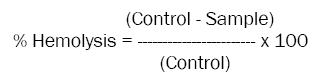
All the assays were performed in triplicates of two to three independent experiments. IC50 values for cytotoxicity tests werederived from a nonlinear regression analysis (curvefit)based on sigmoid dose response curve (variable) andcomputed using Graph Pad Prism 5 (Graphpad, SanDiego, CA, USA) [37,38].
To verify the possible anti-proliferative effect of above plant extracts as a first step toward the development of novel putative anticancer agents, we tested methanol extract of S.indicum (Fruit), R.succedanea (Stem),R.emodi (Resin) andG.gummifera (Resin/Stem)on inhibition and proliferation for check their capability to inhibit cell growth or viability on of DU145, PC-3, H1975, HCT116 and A375cancer cell lines at concentration of 2-128 μ g/ml. Proliferation of these cells was significantly inhibited by above extractsin a concentration-dependentmanner for 24h, as shown in Figure 1. All the four extracts inhibited 70-75 % growth of DU145 (A) cells and the IC50 of S.indicum(8.482 μ g/ml), R.succedanea (24.5 μ g/ml),R.emodi (28.24 μ g/ml) and G.gummifera (28.61 μ g/ml). Whereas the R. succedaneaand R.emodi inhibit 80-90% growth of PC-3 (B) cells and the IC50 of R succedanea (11.04 μ g/ml) and R emodi (11.05 μ g/ml). But S. indicumand G. gummiferainhibit 40-50% growth of PC-3 cells and the IC50 ofS.indicum(11.18 μ g/ml)and G.gummifera(23.03 μ g/ml) respectively. In H1975 (C) cells, S.indicum inhibit 80-90 % whereas R.succedanea, R.emodi and G.gummiferainhibit 40-65 % of growth and the IC50 of S.indicum(9.03μg/ml), R.succedanea (7.71μg/ml),R.emodi (15.95μg/ml) and G.gummifera (11.71μg/ml). About 80-90 % of growth was inhibited in HCT116 (D) cells by all these extracts and it’sIC50 of S.indicum(17.58μg/ml), R.succedanea (8.87μg/ml),R.emodi (19.31μg/ml) and G. gummifera (11.67μg/ml). Finally S.indicum inhibit 75-80 % and remaining extracts inhibit 90-95 % growth of A375 (E) cells and it’sIC50 of S.indicum(27.94μg/ml), R.succedanea (13.13μg/ml),R.emodi (11.05μg/ml) and G.gummifera (7.81μg/ml). Therefore, our results concluded that methanolic extracts of these plants showed strong and broad-spectrum anticancer activity.
Cells were treated with various concentrations (0, 2, 4, 8, 16, 32, 64 and 128 μg/ml) of above mentioned plant extracts for 24 hrs grown in a serum free media. The percentage of cell death induced was determined using the MTT assay and each data represents the mean of threeindependent experiments.
Human erythrocytes were incubated with various concentrations (0, 2, 4, 8, 16, 32, 64 and 128 μg/ml) of above mentioned plant extracts at 370C water bath for 60 min. The absorbance of the resulting supernatant was measured at 540 nm by spectrophotometer (JENWAY 6305 UV/Vis.) to determine the extent of hemolysis. The percentage of haemolysis was calculated by the equation% Hemolysis = [(AControl– ASample) / AControl] x 100. All analyses are the mean of two independent experiments.
Haemolytic assay method is suited to evaluate the haemocompatibility of biomaterials and medical devices according to the international standard ISO 10993-4:2002.As these methanolic extracts of S indicum, R succedanea, R emodiand G gummifera showed anticancer activity in above mentioned human cancer cells, here we have tested 0-128 μg/ml of S. indicum, R.succedanea, R. emodiand G. gummifera extracts effecton human erythrocytes as shown in figure 2. None of the concentrations of the S.indicum extracts showed any visible hemolysis activity; R.emodi extracts showed 2-9.5% hemolysis in a concentration dependent manner but R. succedanea and G. gummifera extracts showed very minimum level of (< 4%) hemolysis at the concentration of 64-128 μg/ml.
This study evaluated the cytotoxic activities of methanol extractsof S. indicum, R.succedanea,R. emodiand G. gummiferainDU145, PC-3, H1975, HCT116 and A375 cancer cell lines and with its IC50 shown in the table 1. The different parts (fruits, leaves, roots) of S. indicum were traditionally used in the treatment of various disorders as mentioned in the above section.In our study S. indicumextract inhibited thegrowth ofDU145, PC-3, H1975, HCT116 and A375 cancer cells and with the IC50concentrations of 8.48, 11.18, 9.03, 17.58 and 27.94μg/ml respectively (Fig 1 and Table 1).Bioactive component of Indiosides isolated from S. indicum have dose-dependent inhibitory effect on proliferation of Bel-7402 cells, and can induce cell apoptosis through mitochondria-dependent pathway [39]. Solavetivone1, a component of S. indicum exhibited cytotoxic to OVCAR-3 cells [40]. Bio actives of beta-Sitosterol (SI-0), beta-sitosterolglucoside (SI-1), dioscin (SI-2), methyl protoprosapogenin A of dioscin (SI-3), methyl protodioscin (SI-4) and protodioscin (SI-5) were isolated from the whole plant of Solanumindicum L and showed cytotoxicity on seven cancer cell lines. The ans SI-3, 4 and 5 showed a tumor inhibitory effect in vivo in C6 glioma cells and in addition SI-2 had an inhibitory effect on the DNA synthesis of C6 glioma cells at 10 micrograms/ml [41].
R. succedanea L, plant is locally known as Kakrasingi and in Indian ethno medicine it has been used as ayurvedic remedy for diarrhoea and dysentery [13]. It also has been reported to possess antiviral [14], antibacterial [15], and cytotoxic properties [16]. Here R. succedaneaextractexhibited the growth of DU145, PC-3, H1975, HCT116 and A375 cancer cells and with the IC50concentrations of 24.5, 11.04, 7.71, 8.87 and 13.13μg/ml respectively (Fig 1 and Table 1). Two new antioxidative and cytotoxic compounds, 10'(Z), 13'(E), 15'(E)-heptadecatrienylhydroquinone (1) and 10'(Z), 13’ (E)-heptadecatrienylhydroquinone (2), as well as the known 10'(Z)-heptadecenylhydroquinone (3), were isolated from an EtOH extract of the sap of Rhussuccedanea exhibited antioxidative and cytotoxic activities against five cancer cell lines [42].
R. emodiextract had cytotoxic effect onthe growth of DU145, PC-3, H1975, HCT116 and A375 cancer cells and with the IC50concentrations of 28.24, 11.05, 15.95, 19.31and 11.05μg/ml respectively (Fig 1 and Table 1). Methanolic extract of R. emodishowed concentration-dependent cytotoxicity in MDA-MB-435S and Hep3B cell lines due to presence of phenolic compounds such as β-Resorcylic acid, Daidzein-8-O-glucoside (puerarin), Daidzein, Quercetin and Flavonol in the R. emodi [43]. Emodin (1) is the major bioactive compound of several herb species, which belongs to anthraquinoneclass of compound, was isolatedfrom the roots of R emodi exhibited antiproliferative activities against HepG2 and MDA-MB-231 cells [44].
Finally G.gummiferaextract also inhibited the growth of DU145, PC-3, H1975, HCT116 and A375 cancer cells and with the IC50 concentrations of 28.61, 23.03, 11.71, 11.67 and 7.816μg/ml respectively (Fig 1 and Table 1).The gum of G.gummifera possesses number of medicinal properties which include anthelmentic, antispasmodic, anti-epilepticcardiotonic and antioxidant potential mentioned in the above section. Dikamaliartane-A, a Cycloartanes isolated from gum resin, G. gummifera had goodin vitro and in vivo anti-cancer activity in HeLa(cervical cancer) and MCF-7(breast cancer) cell lines [45].
Overall our results suggested that the presence of bioactive constituents in the methanolic extractsof S. indicum, R.succedanea, R. emodiand <G. gummifera significantly inhibit the growth of prostate, lung, colorectal and skin carcinoma and induce cell apoptosis through mitochondria-dependent pathway. However we can conclude that all these extracts not only inhibit specific type of cancer cell growth but also inhibit the most type of cancer cell growth.
The hemolysisassay determine whether the methanolicextractsof S. indicum, R.succedanea,R. emodiand G. gummiferacrosses the red blood cell (RBC) membrane, interacting with hemoglobin and initiates a series of reactions, resulting in RBC lysis (haemolysis) [34]. Except S. indicum, remaining extractsexhibited concentration dependenthemolysis activity shown in the figure 2. Most characteristic property of bound phenolic compounds,saponins and other bioactive constituent are their ability to cause haemolysis and since there are presence in these extracts showed haemolysis effect.
According to the American National Cancer Institute, the IC50value to consider a crude extract promising for developmentof anti-cancer drug(s) is lower than a limit threshold of30μg/ml [43]. In our study all S. indicum, R.succedanea,R. emodiand G. gummiferaextracts showed significant IC50value less than the 28μg/ml in above different types of cancer cell growth. All these extracts can thus serve as potential sourcefor anti-cancer compounds.
In conclusion, the results obtained indicated that the methanolic extract ofS. indicum, R.succedanea,R. emodiand G. gummifera not only inhibit specific type of cancer cell growth but also inhibit the most type of cancer cell growth. Medicinal plants are natural products and may have therapeutic potentials; however, being natural does not make them automatically safe. Further work is progress in our laboratory to evaluate their formulation effective both In-vitro and In-vivo anticancer potential and in-depthmechanistic aspects.