e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Damin Liang1, Xiaoju Cheng2, Ziping Zhang1, Zhengjiu Yang1, Tingchao Li3*, Peng Tian3,2*
1Department of Medical Technology, Zunyi Medical College, Zunyi, China
2Scientific Research Center, The Third Affiliated Hospital of Zunyi Medical University, Zunyi, China
3Department of Pathology, The Third Affiliated Hospital of Zunyi Medical University, Zunyi, China
Received: 01-Jun-2023, Manuscript No. JPPS-23-100885; Editor assigned: 05-Jun-2023, Pre QC No. JPPS-23-100885 (PQ); Reviewed: 19-Jun-2023, QC No. JPPS-23-100885; Revised: 26-Jun-2023, Manuscript No. JPPS-23-100885 (R); Published: 03-Jul-2023, DOI: 10.4172/2320-1215.12.2.004
Citation: Liang D, et al. Induction of DNA Damage, Cell Apoptosis and Cell Cycle Check Point by the Usage of Kaempferol. RRJ Pharm Pharm Sci. 2023;12:004
Copyright: © 2023 Liang D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Purpose: Drug resistance is the main cause of chemotherapy failure in hepatocellular carcinoma. Kaempferol (KAE) is a natural flavonoid compound, which has a certain chemo-sensitivity enhancement effect. However, the potential molecular mechanism of KAE reversing drug resistance in hepatocellular carcinoma remains unclear.
Methods: RT-qPCR was used to evaluate the interference effect of siDNAPKcs. RT-qPCR and WB assays were used to detect the mRNA and protein expression of DNA damage repair related genes (γ-H2AX, DNA-PKcs, Artemis) and drug delivery pump gene (P-gp). Flow cytometry was used to detect cell cycle and apoptosis.
Results: In this study, we found that KAE significantly increased the mRNA and protein levels of γ-H2AX, and down-regulated the mRNA and protein levels of DNA-PKcs and Artemis, on the other hand, it also down-regulated the mRNA and protein levels of P-gp, and ultimately jointly promoted the DNA damage, cell apoptosis, and cell cycle arresting in the G2/M phase of drug-resistant Bel-7402/5-Fu cells. Mechanically, KAE mainly promoted the degradation of DNA-PKcs through ubiquitin proteasome pathway, downregulated the protein level of DNA-PKcs, inhibited the DNA-PKcs/Artemis pathway, and promoted DNA damage, induced cell apoptosis and cell cycle arresting.
Conclusion: KAE may be used as a sensitizer for clinical treatment of chemotherapy resistance of HCC, and inhibition of DNA-PKcs may also become a new strategy and target for the treatment of HCC.
Kaempferol; Drug-resistant cells Bel-7402/5-Fu; DNA-PKcs; Apoptosis
Hepatocellular Carcinoma (HCC) is a global malignant tumor with high mortality, characterized by easy metastasis, easy recurrence, refractory treatment and poor prognosis [1,2]. More than 750,000 people in worldwide suffer from hepatocellular carcinoma every year, and almost half of which are in China [3]. A large number of clinical data reveal that HCC is prone to multi-drug resistance after metastasis to most chemotherapeutic drugs (sorafenib, doxorubicin, 5-fluorouracil, platinum-containing anticancer drugs, camptothecin and gemcitabine, etc.), surgery and radiotherapy are also not available [4-6]. Therefore, the development of HCC-targeted drugs or chemo sensitizers has become a new direction for HCC treatment.
Multi-Drug Resistance (MDR) has become the biggest obstacle to the success of cancer chemotherapy, and its mechanism is very complex and still unclear [7]. DNA damage repair is one of the most widely studied molecular mechanisms of tumor resistance. DNA Double-Stranded Breaks (DSBs), the most serious type of damage, can lead to cell death due to loss of division and proliferation, which is an important mechanism for most anticancer drugs to kill tumor cells [8,9]. DNA-dependent kinase catalytic subunits (DNA-PKcs) are the core components of the Nonhomologous End-Joining (NHEJ) pathway, and play an important role in many DNA damage repair pathways. DNA-PKcs has emerged as an attractive therapeutic target in various cancer treatments, especially in chemo-resistant [10,11]. Targeted inhibition of DNA-PKcs proved to be an effective radiosensitizer in vitro, such as NU7441 (DNA-PKcs inhibitor) showing sufficient radiosensitization effects than etoposide, doxorubicin or ionizing radiation [12]. Furthermore, AZD7648, a specific DNA-PKcs inhibitor, showed potent sensitization to radiation or doxorubicin in a patient-derived xenograft model [13]. Therefore, inhibitors of DNA-PKcs show great application prospects in anti-tumor resistance.
These small molecule compounds of DNA-PKcs inhibitors have unstable metabolism in vivo, such as short serum half-life, poor solubility, etc., which limit their application. Therefore, exploring or developing better DNA-PKcs inhibitors is the key to obtain effective anti-tumor resistance drugs. Kaempferol (KAE) is a natural flavonoid with anti-tumor, antibacterial, anti-inflammatory, antioxidant, cardioprotective and other effects [14,15], may act as a potential DNA-PKcs inhibitor. KAE can reduce the expression of DNA repair-related proteins (14-3-3σ, DNA-PK, p-ATM, etc.) in HL-60 cells, induce DNA damage, and then affect the progression of human promyelocytic leukemia [16]. Up to now, however, the regulatory relationship between KAE and DNA-PKcs in hepatocellular carcinoma has not been reported and remains to be investigated. In addition, KAE can trigger apoptosis, cell cycle arresting, and inhibit the progression of breast cancer, colorectal cancer and leukemia [17-19]. KAE can also increase the chemosensitivity of hepatoma cells to sorafenib, and has a certain chemo-sensitization effect [20]. These studies suggest that KAE may act as a sensitizer for chemotherapy resistance of hepatoma cells, promote cell apoptosis and cell cycle arresting, and inhibit the development of hepatocellular carcinoma.
In this study, we investigated whether kaempferol promotes cell apoptosis and cell cycle arresting in drug-resistant hepatocellular carcinoma cells and its underlying molecular mechanisms. Our results found that kaempferol can down-regulate the protein content of DNA damage repair factor DNA-PKcs through ubiquitination pathway, increase DNA damage, and promote apoptosis and cell cycle arresting in Bel-7402/5-Fu cells, and then affect the growth of cells, which can provide a new strategy and target for clinical treatment of HCC chemotherapy resistance.
Chemicals and reagents
Fetal Bovine Serum (FBS) and RPMI 1640 medium were obtained from Invitrogen Corporation (Carlsbad, CA, USA). 5-Fluorouracil (5-Fu) and kaempferol (KAE) were purchased from Solarbio (Beijing, China). Cycloheximide (CHX, ab120093) and MG132 (ab141003), Chloroquine (CQ, ab142116) were purchased from MedChemExpress (Radnor, PA, USA).
Cell culture
Bel-7402/5-Fu cells were donated to Professor Fan Fang, School of Basic Medicine, Zunyi Medical University. The drug-resistant Bel-7402/5-Fu cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum at 37ºC in 5% CO2 incubator. At the same time, 5-Fu (2 μg/mL) was added into the medium to maintain cell resistance.
Cell transfections
For DNA-PKcs gene knockdown, Bel-7402/5-Fu cells were transfected with three different siRNAs of DNA-PKcs (siRNA-1664, siRNA-2142 and siRNA-3785, synthesized in Genepharma, Shanghai, China). The negative control siRNA (si-NC) sequence does not pair with any human RNA sequence. Their sequences were shown in Table 1. When the density of Bel-7402/5-Fu cells was 70-80%, the cells were pretreated with fresh medium without serum for 3 h to starve the cells. Then, the siRNA of DNA-PKcs was transfected into cells with Lipofectamine 3000 transfection reagent (Cat. No. L3000001; Thermo Fisher Scientific, Waltham, MA, USA). After transfection, the cells were cultured in 37℃ incubator for 24 h. RT-qPCR and western blot analysis were used to verify the transfection efficiency.
| Name | Sense (5'-3') | Antisense (5'-3') |
|---|---|---|
| siRNA-2142 | GCAAAGAGGUGGCAGUUAATT | UUAACUGCCACCUCUUUGCTT |
| siRNA-3785 | GAACAUGGCAGGAGAGAAUTT | AUUCUCUCCUGCCAUGUUCTT |
| siRNA-1664 | GUGAAUUCCUCCAGUGAAATT | UUUCACUGGAGGAAUUCACTT |
| si-NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Table 1. RNA sequences used for knockdown of DNA-PKcs gene.
Cell cycle and cell apoptosis analysis
The Bel-7402/5-Fu cells were pretreated with KAE or si-DNA-PKcs (siRNA-1664) for 12 hrs, and then cells were collected and resuspended. Cells (1 × 104 cells/mL) are absorbed and placed in 96 well plates (200 μL/well). Cells were collected after 12 hrs, the effect of KAE and DNA-PKcs on cell cycle and cell apoptosis rate of Bel-7402/5-Fu cells were detected by cell cycle and apoptosis detection kit (C1052; Beyotime Biotchnology, Shanghai, China) according to the manufacturer’s instructions.
Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) analysis
Total RNAs were extracted from Bel-7402/5-Fu cells using the TRIzol reagent (Cat. No. 12183555; Thermo Fisher Scientific, Waltham, MA, USA). Total RNAs were reversely transcribed to cDNA using the Reverse Transcription Kit (Cat. No. 6210A; Takara, Otsu, Japan). Primers were purchased from Sangon Biotech (Shanghai, China) and their sequences were listed in Table 2. RT-qPCR was performed on CFX96 system (Bio-Rad, Hercules, CA, USA). GAPDH mRNA was used as an internal control. The RNA expression fold change was calculated using the 2−ΔΔCt method. Table 2. RNA sequences used for knockdown of DNA-PKcs gene.
| Gene | Forward (5'-3') | Reverse (5'-3') |
|---|---|---|
| DNA-PKcs | CCTGGCCGGTCATCAACTG | AGTAAGGTGCGATCTTCTGGC |
| H2AX | CTACTCCGAACGAGTCGGG | GATGGTCACGCGACCTAGAAG |
| Artemis | TATGCCGGTCTTCCCAAAGTA | GTGAAAAGTTTCCGGGTATGGA |
| P-gp | TGACCCGCACTTCAGCTAC | GGGCTTCCCGATGATGTCG |
| GAPDH | TGTGTCCGTCGTGGATCTGA | GCAGCTGTGACACACAGTA |
Table 2. RNA sequences used for knockdown of DNA-PKcs gene.
Western blotting assay
After the experimental treatment, Bel-7402/5-Fu cells were removed from the culture medium, washed with pre-cooled sterile PBS for three times, and then directly added 500 μL of pre-cooled RIPA lysate (P0013B, Beyotime Biotchnology, Shanghai, China), cracked on ice for 30 min, and quantified according to BCA quantitative kit. And then proteins were separated using SDS-PAGE and analyzed by western blot assays, as described previously [21]. The primary antibodies included anti-mouse γ-H2AX (Abcam, ab26350, 1:1000, UK), anti-rabbit DNA-PKcs (Abcam, ab70250, 1:1000, UK), anti-rabbit Artemis (Abcam, ab138411, 1:800, UK), anti-rabbit P-gp (Abcam, ab261736, 1:1000, UK), anti-GAPDH (Abcam, ab8245, 1:10000, UK). The secondary antibodies were goat anti-rabbit IgG (HRP) (Abcam, ab6721, 1:10000, UK) and goat anti-mouse IgG (HRP) (Abcam, ab6789, 1:10000, UK). Protein bands were visualized using beyoECL Reagent (P0018FS; Beyotime Biotchnology, Shanghai, China) with Chemi-Doc imaging system (Bio-Rad, Hercules, CA, USA).
Protein stability analysis
To measure protein stability, Bel-7402/5-Fu cells were treated with KAE (8 μM) for 24 hrs, and then added 100 μg/mL Cycloheximide (CHX) to block protein translation. After CHX was treated at 0, 2 or 4 hrs, proteins were extracted and their expression levels were evaluated by western blotting. GAPDH was used as the internal control.
In vitro ubiquitination assays
Bel-7402/5-Fu cells were treated with 10 μM MG132 or 50 μM CQ to block protein degradation. The ubiquitinated DNA-PKcs (DNA-PKcs-Ub) in cell lysate was immunoprecipitated by anti-DNA-PKcs (5 μg antibody/500 μg total protein for IP, dilution 1:000 for IB, ab70250, Abcam, UK). Briefly, Bel-7402/5-Fu cell lysate was incubated with anti-DNA-PKcs at 4°C overnight with gentle rotation. Then 40 μL magnetic beads A/G (B23201, Bimake, USA) were added to the mixture and incubated at 4°C for 4 hrs with gentle rotation. Subsequently, the immunoprecipitated proteins were separated by SDS-PAGE and DNA-PKcs-Ub was detected by western blotting using anti-Ub (ab134953, 1:1000, Abcam, UK).
Statistical analysis
All experiments were independently performed thrice and data were presented as mean ± standard deviation (mean ± SD). The experimental data and graphs were statistically analyzed and plotted using GraphPad Prism 8.0.1 (GraphPad, USA). The student’s t test or one-way ANOVA followed by Dunnett test was used to evaluate the differences between two groups or among more than two groups, respectively. P<0.05 was considered statistically significant.
KAE promotes DNA damage in Bel-7402/5-Fu cells
KAE induces rearrangement of the MLL gene by promoting Double-Strand Breaks (DSBs) in human primary CD34+ cells [22]. However, whether KAE can induce DNA damage in drug-resistant hepatoma cells remains unclear. Firstly, we evaluated the cytotoxic effect of KAE on drug-resistant Bel-7402/5-Fu cells, and found that with the increase of KAE concentration and treatment time, the cell mortality increased in a time-dependent relationship. Subsequently, we selected KAE at 8.0 μM to treat cells for 24 hrs as the best condition for mechanism analysis (Figure 1A). In Bel- 7402/5-Fu cells treated with KAE, our results showed that KAE significantly up-regulated the mRNA and protein levels of γ-H2AX (DNA damage marker gene), compared with the control group (0.05% DMSO) (Figures 1B-1D). In addition, KAE also significantly down-regulated the mRNA and protein levels of DNA-PKcs, Artemis (DNA damage repair genes), and P-gp (drug delivery pump gene) in Bel-7402/5-Fu cells (Figures 1B-1D). These results suggested that KAE might promote DNA damage by inhibiting the expression of DNA damage repair genes (DNA-PKcs and Artemis), or increasing cell sensitivity to 5-Fu by inhibiting P-gp level in Bel-7402/5-Fu cells (Figure 1A).
Figure 1: Kaempferol promotes DNA damage in Bel-7402/5-Fu cells. Drug-resistance Bel-7402/5-Fu cells were incubated with DMSO (0.05%) or Kaempferol (8.0 μM KAE) for 24 hrs. (A) RT-qPCR analysis of the mRNA levels of genes that function for DNA damage repair genes (γ-H2AX, DNA-PKcs and Artemis), drug delivery pump gene (P-gp)
in KAE-treated Bel-7402/5-Fu cells; (B) Western blot analysis of the protein levels of γ-H2AX, DNA-PKcs, Artemis and
P-gp in KAE-treated Bel-7402/5-Fu cells, with GAPDH as internal standard; (C) Quantitative results of protein relative to GAPDH. Data in (A-C) show mean ± SD, n=3 independent experiments. Student’s t test analysis for (AC), P<0.05 was considered as significant, *P<0.05, **P<0.01.
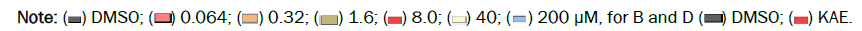
KAE induces cell apoptosis and cell cycle arresting
To further explore whether KAE also could affect the cell cycle and cell apoptosis of Bel-7402/5-Fu cells. The cell cycle of Bel-7402/5-Fu cells was detected by PI single-staining flow cytometry. The results showed that the ratio of G0/G1 phase of Bel-7402/5-Fu cells treated with KAE was significantly decreased (P<0.01), the S phase was basically unchanged (P>0.05), but the G2/M phase was significantly increased (P<0.05), compared with the control group Figure 2A, indicating that KAE could arrest cell cycle at G2/M phase in Bel-7402/5-Fu cells.
Furthermore, the apoptosis rate of Bel-7402/5-Fu cells was detected by Annexin V-FITC/PI double staining flow cytometry. The results showed that the degree of apoptosis of Bel-7402/5-Fu cells was significantly increased after KAE treatment, compared with the control group, and the apoptosis rate was 17.81% (P<0.05) Figure 2B, indicating that KAE promoted cell apoptosis in Bel-7402/5-Fu cells. These results suggested that KAE might induce cell apoptosis and cell cycle arresting in Bel-7402/5-Fu cells (Figure 2A).
Figure 2: Kaempferol induces cell apoptosis and arrests cell cycle. (A) The effect of Bel-7402/5-Fu cell cycle treated with DMSO or KAE was detected by FCM; (B) The effect of Bel-7402/5-Fu cell apoptosis treated with DMSO or KAE was detected by FCM. Significance; Data in (A, B) show mean ± SD, n=3 independent experiments. Student’s t test analysis for (A, B), P<0.05 was considered as significant, ns means no significant, *P<0.05, **P<0.01.

Knockdown of DNA-PKcs induces cell apoptosis and cell cycle arresting
Up to now, many studies have demonstrated that DNA-PKcs inhibitors are radio and chemotherapy-resistant sensitizers for many tumors, such as leukemia, colorectal cancer, etc [23,24]. However, the role of DNA-PKcs in reversing drug resistance in hepatocellular carcinoma remains unclear. Therefore, small interfering RNAs (siRNAs) were used to knockdown DNA-PKcs to explore the effects of DNA-PKcs on cell apoptosis and cell cycle in Bel- 7402/5-Fu cells. It was found that the three interfering sequences (siRNA-1664, siRNA-2142 and siRNA-3785) could significantly down-regulate the mRNA and protein levels of DNA-PKcs (Figures 3A and 3B). SiRNA-1664 was then selected for subsequent experiments.
The results showed that, compared with the control group (si-NC), the cell cycle of Bel-7402/5-Fu cells did not change in G0/G1 phase (P>0.05), but significantly increased in S phase (P<0.05), decreased in G2/M phase (P<0.05) after knockdown of DNA-PKcs (Figure 3C). It is suggested that knockdown of DNA-PKcs could block the cell cycle in G2/M phase. Furthermore, knockdown of DNA-PKcs significantly increased cell apoptosis of Bel- 7402/5-Fu cells, compared with the control group (si-NC) (P<0.05) (Figure 3D). These results suggested that inhibiting or interfering with DNA-PKcs might serve as a potential target for the treatment of drug resistance in HCC (Figure 3A).
Figure 3: Knockdown of DNA-PKcs induces cell apoptosis and arrests cell cycle. Bel-7402/5-Fu cells were
transfected with control siRNA (si-NC) or DNA-PKcs siRNAs (siRNA-1664, 2142 or 3785) for 24 hrs. (A-B) The mRNA and protein levels of DNA-PKcs was analyzed by RT-qPCR and WB assays, with GAPDH as internal standard; (C) the effect of Bel-7402/5-Fu cell cycle treated with siRNA-1664 was detected by FCM; (D) the effect of Bel-7402/5-Fu
cell apoptosis treated with siRNA-1664 was detected by FCM. Significance; Data in (A-D) show mean ± SD, n=3
independent experiments. Student’s t test analysis for (A-D), P< 0.05 was considered as significant, *P<0.05, **P<0.01.

KAE induces cell apoptosis and cell cycle arresting via DNA-PKcs
We found that KAE induces DNA damage, cell apoptosis and cell cycle arresting, while DNA-PKcs can also induce cell apoptosis and cycle arresting in Bel-7402/5-Fu cells. Whether KAE may affect cell apoptosis and cell cycle through DNA-PKcs remains unclear. Therefore, KAE-treated cells and simultaneously knockdown by DNA-PKcs to explore its internal relationship. Our results showed that KAE significantly up-regulated the mRNA and protein levels of γ-H2AX, but significantly down-regulated the mRNA and protein of P-gp, DNA-PKcs and Artemis, compared with the control group (Figures 4A-4C). However, knockdown of DNA-PKcs further promoted the effect of KAE in Bel- 7402/5-Fu cells (Figures 4A-4C). These results suggested that KAE might promote DNA damage, cell apoptosis and cell cycle arresting by down-regulating DNA-PKcs (Figure 4A).
Figure 4: Kaempferol induces cell apoptosis and cell cycle arrest via DNA-PKcs. Bel-7402/5-Fu cells were incubated with DMSO or KAE (8.0 μM) for 12 hrs and were then transfected with control siRNA (si-NC) or si-DNA-PKcs (siRNA-
1664) for 12 hrs. (A) The mRNA levels of γ-H2AX, DNA-PKcs, P-gp and Artemis was analyzed by RT-qPCR assays; (B)
The protein levels of γ-H2AX, DNA-PKcs, P-gp and Artemis was analyzed by RT-qPCR assays, with GAPDH as internal standard; (C) Quantitative results of protein relative to GAPDH. Significance; Data in (A-C) show mean ± SD, n=3
independent experiments. Student’s t test analysis for (A, C), P<0.05 was considered as significant, *P<0.05, **P<0.01.
![]()
KAE promotes ubiquitination and degradation of DNA-PKcs
We found that KAE could significantly down-regulate the protein levels of DNA-PKcs, but the molecular mechanism has not been elucidated. Therefore, cycloheximide (CHX, 10.0 μM) was added to prevent protein translation, and the effect of KAE on the protein stability of DNA-PKcs at different time periods (0, 2, 4 hrs) was investigated in KAEtreated Bel-7402/5-Fu cells. The results showed that, compared with the control group, the stability of DNA-PKcs protein was significantly reduced after KAE treatment, indicating that KAE could promote the degradation of DNAPKcs protein (Figure 5A).
Figure 5: Kaempferol down-regulated DNA-PKcs expression level by promoting its proteasomal degradation. (A) DNA-PKcs protein level was detected at indicated time in DMSO or 8.0 μM KAE-treated Bel-7402/5-Fu cells after addition of 10 μM CHX, GAPDH was used as internal standard; (B) DNA-PKcs protein level was analyzed in Bel-7402/5-Fu cells treated with 8.0 μM KAE for 18 hrs and then with DMSO, 10 μM MG132, or 50 μM CQ for 6 hrs;
(C) DNA-PKcs-Ub level was determined in Bel-7402/5-Fu cells treated with 8.0 μM KAE for 18 hrs and then with
DMSO or 10 μM MG132 for 6 hrs. DNA-PKcs level in input was also analyzed, with GAPDH as internal standard. Significance; Data in (A-C) show mean ± SD, n=3 independent experiments. Student’s t-test for (A); and P<0.05 was considered as significant, *P<0.05, **P<0.01.

It has been found that proteins are degraded mainly through two important pathways: the ubiquitin-proteasome pathway and the autophagy-lysosome pathway, which can be specifically blocked by the treatment of MG132 or CQ, respectively. Our results found that DNA-PKcs protein expression was significantly reduced in KAE-treated cells compared with the control group (Figure 5B). When MG132 or CQ was added to prevent the degradation of proteasome or autophagy pathway, the expression of DNA-PKcs protein in the MG132 group was significantly increased, while the change of DNA-PKcs protein in the CQ group was not significant, compared with the control group (Figure 5B). These results suggested that KAE down-regulated DNA-PKcs protein levels mainly through the ubiquitin-proteasome pathway in Bel-7402/5-Fu cells.
To further confirm, KAE down-regulated DNA-PKcs protein level through the ubiquitin-protease pathway. In this study, the level of DNA-PKcs ubiquitination was detected by Co-IP assay. The results showed that KAE could significantly down-regulate DNA-PKcs protein level and increase DNA-PKcs ubiquitination (DNA-PKcs-Ub) level in KAE-treated cells, compared with the control group (Figure 5C; Lane 2). After the addition of MG132 to block the ubiquitination pathway, DNA-PKcs protein levels were significantly rescued, while also promoting the accumulation of DNA-PKcs-Ub level (Figure 5C; Lane 3), suggesting that KAE might be activated by ubiquitin-protease pathway, which increased the ubiquitination and promoted the degradation of DNA-PKcs, finally down-regulated the protein levels of DNA-PKcs (Figure 5C).
Hepatocellular carcinoma is one of the most lethal gastrointestinal malignancies, and more than 50% of liver cancer patients are diagnosed at an advanced stage [25,26]. Currently, surgery and chemotherapy are the main preferred treatment strategies for advanced liver cancer. However, with the metastasis and drug resistance of advanced liver cancer, few effective chemotherapeutic drugs are available for liver cancer treatment [27]. 5-Fu, a DNA-targeted cell cycle-specific drug, has failed treatment due to the development of resistance, but it remains the recommended chemotherapeutic drug [28]. Therefore, new therapeutic strategies are urgently needed to improve the efficacy of 5-Fu chemotherapy and overcome drug resistance. Traditional Chinese medicine is rich in resources and has many targets, which can target the complex characteristics of chemotherapy resistance mechanism [29]. The reported active components of traditional Chinese medicine with resistance reversal effect include kaempferol, ginsenoside, astragaloside IV, etc [30-32]. Our results found that kaempferol derived from traditional Chinese medicine could promote cell apoptosis and cell cycle arresting of Bel-7402/5-Fu cells Figure 6, which may provide an effective method for the treatment of drug-resistant liver cancer patients.
Figure 6: Mechanism of Kaempferol induced DNA damage, cell apoptosis and cell cycle arrest in Bel-7402/5-Fu cells. Kaempferol promoted the ubiquitination of DNA-PKcs and down-regulate the protein level of DNA-PKcs. Moreover, kaempferol significantly up-regulated γ-H2AX level, and down-regulated Artemis level, and ultimately jointly promote the DNA damage, apoptosis, and cell cycle arrest in drug-resistant Bel-7402/5-Fu cells.
Kaempferol is a tetrahydroxy flavonoid found in many fruits, vegetables and medicinal plants [33]. In vitro studies and some animal tests have demonstrated that kaempferol has antibacterial, antioxidant, antitumor, and cardioprotective activities. At the same time, kaempferol can also be combined with other chemotherapeutic drugs to improve the efficacy of chemotherapy and prevent multidrug resistance. For example, the combination of kaempferol and 5-Fu can induce cell apoptosis and cell cycle arresting in 5-Fu resistant colon cancer cells LS174, reducing the cell migration and invasion [34]. Kaempferol cooperates with DOX to inhibit the proliferation, migration and invasion of liver cancer cells Huh-7 and HepG2 by down-regulating PI3K/mTOR/MMP pathway [35]. The research of kaempferol in tumors is mainly aimed at single drug use or synergistic effect with other chemotherapeutic drugs, but less research on liver cancer drug resistance and the specific molecular mechanism is also unclear. Our study found that kaempferol could promote DNA damage and induce cell apoptosis and cell cycle arresting in Bel-7402/5-Fu cells by down-regulating DNA-PKcs protein level.
DNA-PKcs is a regulator of radiation-induced DNA damage repair and is considered as a novel target for intervention in cancer therapy. Knockdown of DNA-PKcs significantly increases tumor susceptibility in mouse models. In addition, targeting DNA-PKcs with various inhibitors can also effectively enhance radiotherapy and has been used as an effective strategy to improve the prognosis of cancer patients [36]. However, it is not clear whether inhibition of DNA-PKcs affects the growth of drug-resistant liver cancer cells. Our results showed that interfering with DNA-PKcs could promote DNA damage and induce apoptosis and cell cycle arresting in Bel-7402/5-Fu cells. Therefore, inhibiting or interfering with DNA-PKcs may be a potential chemotherapeutic strategy for drug-resistant tumors.
Generally, intracellular proteins undergo degradation through autophagy-lysosome and ubiquitin-proteasome pathways. Studies have found that DNA-PKcs can be highly ubiquitinated to degrade cytoplasmic DNA-PKcs, reduce the content of cytoplasmic and nuclear DNA-PKcs, and then inhibit the functions of innate immunity such as macrophages and Natural Killer (NK) cells [37]. Doxorubicin or neocarcinstatin treatment can up-regulate the expression of RNF144A, promote the ubiquitination of DNA-PKcs, and reduce the level of DNA-PKcs, thereby inducing apoptosis of human colon cancer cells HCT116 [38]. Metastasis-associated 1 (MTA1) mediated DNA-PK degradation via the proteasome and inhibited H1.2 phosphorylation, thereby promoting hepatocellular carcinoma progression in HuH6 cells [39]. Based on this, we speculate that kaempferol may also mediate DNA-PKcs degradation via the proteasome in Bel-7402/5-Fu cells. The results confirmed that kaempferol could promote the ubiquitination of DNA-PKcs and down-regulate the protein level of DNA-PKcs. Therefore, down-regulation of DNAPKcs protein levels might provide an efficient approach to promote cell death by promoting the proteasomal degradation pathway of DNA-PKcs (Figure 6).
We found that kaempferol could significantly increase DNA-PKcs ubiquitination, down-regulate DNA-PKcs protein level. Moreover, kaempferol could significantly up-regulated γ-H2AX level, and down-regulated Artemis and P-gp level, and ultimately jointly promoted DNA damage, induced apoptosis and cell cycle arresting in drug-resistant Bel- 7402/5-Fu cells, thereby affecting cell growth. This study could provide a new strategy and target for clinical treatment of HCC chemotherapy resistance.
This work was supported by the Joint project of Zunyi Science and Technology Bureau (No. ZSKH-SZ (2018)41, ZSKH-HZ (2020)117, ZSKH-HZ (2022)43, ZSKH-HZ (2022)151), the Science and Technology Plan Project of Guizhou Province (No. ZK (2021)494) and the Science and Technology Plan Project of Guizhou Health Commission (No. gzwjkj2019-1-045). We thank Professor Fang Fan for the Bel-7402/5-Fu cells used in this study.
The authors declare that they have no competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]