ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Deepak Kumar M ,Karthick M , Dineshbabu D, Srikanth P , Ramachandran M G Department of Mechanical Engineering, Velammal Engineering College, Chennai, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This project investigates the use of dimethyl ether in diesel engines as alternative fuels. DME is produced by the conversion of various feed stock such as natural gas, coal, oil residue sand bio-mass. Diesel – Dimethyl Ether (BDE) wastested in a 4-cylinder direct-injection diesel engine to investigate the performance and emission characteristic softheengineunderfiveengineloadsatthemaximumtorque.DME’sdieselengine- compatible properties are its high cetane number and low auto-ignition temperature. In addition, it’s simple chemical structure and high oxygen contentresultinsoot- re ecombustionin engines. The dimethylether issued as an alternative fuel mean stone hence the engine combustion. The Diesel 100% (only diesel), and DDE50 (diesel 50% and Dimethyl lether50%) and DME 100% (only dimethylether) were tested in the engine. There sults indicate that when compared with neat diesel, the engine performance increased and emission level decreased with adding the dimethylether with diesel. In comparison with neat diesel, the DDE50 blends have50% higher brake thermal efficiency (BTE). The experimental results showed that the CO, HC and NOx emission is decreased for all DDE blends. The brake specific fuel consumption (BSFC) decreased for all DDE blends compared to neat diesel fuel
Keywords |
| Di methyl ether, diesel, alternative fuel, compression ignition engine |
INTRODUCTION |
| Di-methyl ether (DME) is aliquefiedgaswithhandlingcharacteristicssimilartothoseofliquefied petroleum gas (LPG) [1]. It can be produced from a variety offered-stock such as natural gas, crude oil, residual oil, coal, waste products and biomass. Many investigations have been carried out on DME to determine its suitability for use as a fuel in diesel- cycle engines [1,2]. DME has the appearance of an excellent, efficient alternative fuel for use in a diesel engine, with almost smoke-free combustion. This is not only because of it low auto-ignition temperature and its almost instantaneous vaporization when injected into the cylinder, but al so because of its high oxygen content (around35%bymass) and the absence of C– C bonds in the molecular structure[1,2]. With a properly designed DME injection and combustion system, nitrogen oxides (NOx) emissions can also meet ultra-low emission vehicle (ULEV) limits[3]. The well-towheels energy efficiency of heavy and light-duty DME-fuelled vehicles is projected to be 22.5%and19%,respectively[4].This is comparable to LPG and compressed natural gas(CNG)fuelled vehicles but less than the highest energy efficiency of 26% indirect-injection (DI) dieselfuelledvehicles [4].Ontheotherhand,thewell-towheels carbon dioxide(CO2)emissions ofaDME-fuelled vehiclearecomparable tothoseusingDIdieselor CNGfuelledengines [4].However, anoxidation catalystwouldbenecessary tomeetULEVcarbon monoxide(CO) and hydrocarbon(HC) emission limits[5]. |
| DME was also found to be an excellent gas turbine fuel with emission properties comparable to natural gas[6]. DMEfuelled turbine also allows achieving a significant performance improvement through thermo chemical recuperation with 44% higher power output and an 8% decrease of the specific CO2 emissions compared to the present plant [7]. However, DME is not a suitable fuel for spark- ignition (SI) engines due to it shighcetane number, though the burning velocity is similar to hydro carbon fuels [8]. The easily-induced knock would limit the operation of SI engines. Overall, the key to the development of dedicated low emissions, DME-fuelled engine is the performance and durability of its fuel-injection system [1,2]. In this review, the features considered to be most important in developing the potential for wide spread utilization of DME to reciprocating engines are: the production and properties of DME, the fuel-injection system and the spray characteristics which contribute to the engine’s performance and exhaust emissions. |
DIMETHYLETHER IN BRIEF |
| Di-methyl ether (DME) is alique field gas with handling characteristics similar to those of lique fied petroleum gas (LPG). It canbe produced from a variety of feed-stock such as natural gas, crude oil, residual oil, coal, waste products and bio-mass. Many investigations have been carried out on DME to determine its suitability for use as a fuel in dieselcycle engines. |
| DME has the appearance of an excellent, efficient alternative fuel for use in a diesel engine, with almost smoke-free combustion. This is not only because of it slow auto ignition temperature and its almost instant an when injected to the cylinder, but al so because of its high oxygen content (around35%bymass) and the absence of C–C bonds in the mole collar structure. Witha properly designed DME injection and combustion system, nitrogen oxides (NOx) emissions canal some etultra-lowemission vehicle (ULEV) limits. The well-to-wheels energy efficiency of heavy-and light-duty DME- fuelled vehicles is projectedtobe22.5%and19%, respectively. This is comparable to LPG and compressed natural gas(CNG) fuelled vehicles but lessthanthehighestenergyefficiencyof26%in direct-injection(DI)diesel fuelled vehicles. |
 |
| Production of Dimethyl ether |
| A feasibility study on the production of 99.5% dimethyl ether (DME) is to be performed. The plant is capable of producing 50,000 metric tons of DME per year via the catalytic dehydration of methanol over an acid zeolite catalyst. The goal is to design a grass-roots facility, which safely and efficiently produces DME. DME is used primarily as a propellant. DME is miscible with most organic solvents and it has a high solubility in water. Recently, the use of DME as a fuel additive for diesel engines has been investigated due to its high volatility (desired for cold starting) and high cetane number. |
| C. Properties of Dimethyl ether |
| The key properties of DME and diesel fuel are show ninth table, it as allow carbon-to- hydrogen ratio (C:H) with a chemical formula ofCH3–O-CH3. DME in a gaseousstateis invisible under standard atmospheric conditions (0.1MPaat298K).When it is pressurized above0.5MPa,it condenses to the liquid phase. Gaseous DME is denser than air while liquid DME has a density withers that of water. The vapor pressure is similar to that of LPG and requires the same handling and storage precautions. It dissolves in water up to 6% by mass .However; it is not compatible with most leas tamers due to its corrosiveness, so that careful selection of materials is necessary to prevent deterioration of seals after prolonged exposure to DME. |
 |
EXPERIMENTAL STUDY |
| The experimental setup is shown in Fig. 2. The specification of the engine used is in Table. 2. The engine was connected to an eddy current dynamometer, and a control system was used for adjusting its speed and torque. The engine was run at a constant speed of 1500 rpm. The NOx, CO and HC emission were measured with non-dispersive infrared analysers (NDIR) (Make: HORIBA make Gas Analyser). The gas analyser was calibrated with standard gases and zero gas periodically. Experiments were conducted at the engine speed of 1500 rpm and at five engine loads. At each engine operating mode, experiments were carried out for the diesel (D100), diesel-dimethyl ether namely DDME50, DME100. |
 |
RESULTS & DISCUSSION |
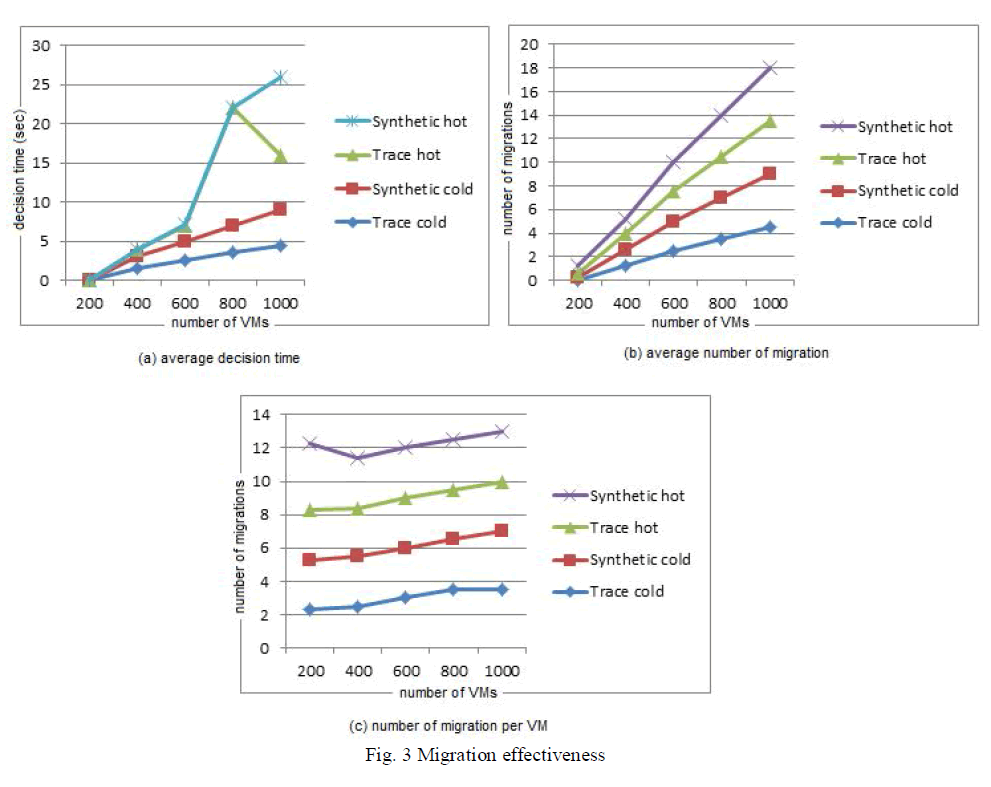
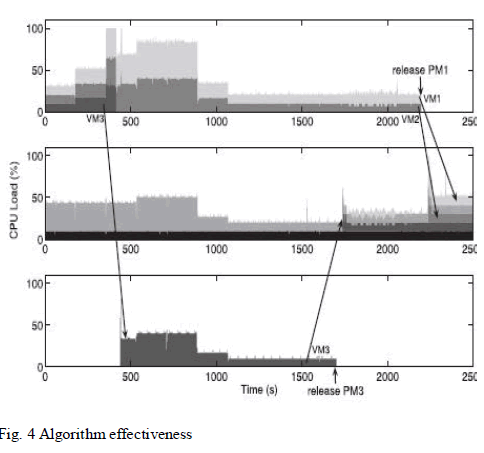
| Fig 3 – 6 depicts the various performance parameters of the compression ignition engine running with diesel and dimethyl ether blends. It can be observed that adding dimethyl ether blend with the diesel fuel gives out almost the same performance as running with diesel fuel. Hence dimethyl ether serves as a suitable alternate fuel to replace a significant amount of the conventional diesel fuel. It can also be observed from Fig 7 – 9 that significant amount of NOx and HC emissions are reduced with the use of dimethyl ether blends. |
 |
 |
 |
 |
CONCLUSION |
| DME is the appearance of an excellent, efficient alternative fuel for use in a diesel engine, with almost smoke-free combustion. This is not only because of its low auto ignition temperature and its almost instantaneous vaporization when injected into the cylinder, but also because of its high oxygen content and the absence of C–C bonds in the molecular structure. With a properly designed DME injection and combustion system, nitrogen oxides (NOx) emissions can also meet ultra-low emission vehicle (ULEV) limits. |
References |
|