E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Department of Pharmacognosy, Al-Ameen College of Pharmacy, Hosur Road, Bangalore- 560 027, Karnataka, India
Received date: 12/06/2014 Accepted date: 27/06/2014
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Diabetes mellitus is a chronic disorder characterized by high blood glucose levels, whose critical management alleviates multiple complications. Ill-managed diabetes often precedes incidence of dyslipidaemia and cardiac diseases. Mangifera indica L (Anacardiaceae) is a popular Indian horticultural tree also used medicinally. Hypoglycaemic and/or antidiabetic activity of leaf has been investigated in various models; however a comparative account of hypoglycaemic potential of mature and tender leaf is not reported; effect of leaves on carbohydrate digesting enzymes also is not reported. The present study is a preliminary report on comparative hypoglycaemic properties of aqueous methanolic extracts of mature and tender leaves of M indica L var. Totapuri in glucose loaded Wistar Albino rats and In-vitro ï¡glucosidase and ï¡ amylase inhibition bioassays. Significant hypoglycaemic activity was observed with both extracts in oral glucose tolerance test at 500mg/Kg b.wt. given orally one hour prior glucose loading. The extracts also showed inhibition of rat intestinal alpha glucosidase ,as well as porcine pancreatic alpha amylase with IC50: 21.03 and 35.73 for mature leaf and 27.16 and 22.01 for tender leaf extracts respectively. Thus the hypoglycaemic potential of mango leaves may be due to inhibition of carbohydrate digesting enzymes. Polyphenols, flavonoids and saponins identified in the extracts may be responsible for their hypoglycaemic activity.
Mangifera indica L, hypoglycaemic, α glucosidase, α amylase, oral glucose tolerance test
Diabetes mellitus is a chronic disease characterized by uncontrolled increase in blood glucose levels beyond homeostatic range due to deficient insulin secretion or reduced responsiveness of tissues to secreted insulin. The aim of antidiabetic therapy is to achieve normoglycaemia to avoid diabetes associated complications such as nephropathy, neuropathy, micro/macroangiopathy, retinopathy and cataract[1]. Postprandial hyperglycaemia is the earliest indicator of deranged glucose metabolism. Control of postprandial hyperglycaemia can lead to a 45% decrease in the incidence of retinopathy and other micro and macro vascular complication.. An important strategy to reduce postprandial hyperglycaemia is to retard absorption of glucose by using inhibitors of carbohydrate digesting enzymes such as α glucosidase and α amylase[ 2,3].
Mangifera indica Linn. (Anacardiaceae) the mango, is a tropical tree grown abundantly in India, most popular for its fruit. Several varieties of the tree made evident by the different mango fruit varieties are available in India and other countries. Leaf of this tree is used as ruminant feed. Its ethnomedicinal uses include treatment of abscesses, tympanitis, colic, diarrhea, liver disorders, tooth decay, coughs and asthma. Hot water extract is taken orally for diabetes in Nigerian medicine. Leaf extracts of this tree has been reported for anti nematodal and anti-viral activity. Mangiferin, a xanthone-C-glucoside is the major compound in mango leaves [4,5]. Phytoconstituents such as indicine, α thujene, 3- carene, ocimene, terpinene, camphene and sabinene are identified from steam distilled leaf oil. Volatile oil from Egyptian mango leaf contains methyl, ethyl, n-propyl, n-butyl, n-amyl and isobutyl alcohols. Triterpene alcohol: indecinol; taraxerone, taraxerol, friedelin, lupeol, galloyl p-hydroxybenzoyl esters of maclurin 3C-glucosides I-IV; galloyl esters of iriflophenone 3C glucosidesV,VI;(-) epicatechin-3-O-gallate, isomangiferin and a new xanthone C-glucoside gallate(VII) along with β-sitosterol have been isolated from leaf[6].
An attempt is made to compare and investigate the antidiabetic activity of mature and tender leaves of Mangifera indica var. Totapuri by testing oral glucose tolerance of the leaf extracts in rats and their α glucosidase and α amylase inhibitory activities in-vitro.
Collection of Plant Materials
The tender and mature leaves of M.indica L. cultivar Totapuri, for the study were collected from healthy trees in the cultivated groves of GKVK, Bangalore. Mature Leaves collected were manually freed of dust and adhering material. Mal-formed leaves, leaves with weevils and those with gals were discarded. Sorted leaves were dried in shade at room temperature for about ten days on mats indoors till they were crisp dry. The so dried leaves were packed and stored in polythene bags and have been found to be free from microbial contamination. Tender leaves were collected during January-February and were shade dried at room temperature indoors.
Sources of α glucosidase and α amylase
Microvillar alpha glucosidase was isolated from healthy adult male rats weighing 200-250g by a method described by Dalquist et al[7]; Porcine pancreatic alpha amylase was procured from sigma chemicals; Acarbose was a gift sample from Ajantha pharma Ltd.
Preparation of the Plant Extracts
Powdered drug material of the leaves were each defatted by refluxation with petroleum ether for 8hrs. The defatted marc was then refluxed with 70% aqueous methanol for 8 hrs. The extracts were filtered, dried using rotary vacuum evaporator, stored in refrigerator prior to use.
Screening for hypoglycaemic activity by oral glucose tolerance test in normoglycaemic rats
Oral glucose tolerance test (OGTT) in normoglycaemic rats was used as preliminary screening test for evaluation of antihyperglycaemic activity [8]. The % inhibition in glucose induced hyperglycemia at 15 minutes in treated animals in comparison with untreated animals after oral glucose administration (1g/kg body weight) was used as a parameter for indication of hypoglycemic property of the screened M. indica extracts.
Wistar Albino rats of either sex weighing 200 to 250 g were divided into three groups of six animals each. The animals were fasted overnight. One hour prior to glucose loading, fasting blood glucose level was determined using Prestige glucometer. The animals of groups II and III were then administered with 500mg/Kg b.wt. of tender and mature leaf extracts respectively. Group I served as control. Animals were loaded with glucose-1g/Kg body weight using 50% glucose solution after 1hr of extract administration. Blood was collected at 15min, 30 min. 1hr, 2hr, 3hr and 4hrs post glucose loading and blood glucose levels were determined using Prestige glucometer-strips. Details of the groups and results are summarized in Table 1. Results were analysed for statistical significance by one way ANOVA.
The % reduction in glucose induced hyperglycaemia was calculated using the equation:
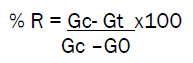
Gc = mean blood glucose level of control group at time at 15min; Gt = mean blood glucose level of treated group at time ‘t’ ; G0 = mean fasting blood glucose level % reduction in glucose induced hyperglycaemia at various intervals after glucose loading is recorded in Table 2.
The handling of animals followed protocols approved by the institutional ethics committee for animal experimentation.
Screening for in-vitro alpha glucosidase inhibitory activity
Isolated rat intestinal α glucosidase was diluted suitably using 80mM Phosphate buffer (pH 7.0) to a protein content of about 0.5g/dl[9]; so diluted enzyme was identified by testing for sucrase and maltase activity. The extracts were tested for maltase inhibitory activity in-vitro using the isolated rat intestinal alpha glucosidase[10]. Briefly rat intestinal α glucosidase with extract of different concentration/acarbose/vehicle buffer made upto 350µl with phosphate buffer pH 7.0 was taken in eppendorff tubes and incubated at 37°C for 30 min.; 500µl of 28mM maltose was added to each of the tubes and incubated for 20 min at 37°C. The enzymatic reaction was stopped by placing the eppendorff tubes in a boiling water bath for two minutes and cooled. 50α of the reaction mixture was pippeted out into separate wells of a microtitre plate, 250µl of glucose oxidase peroxidase reagent was added to each of the wells and incubated for 10 min. at 37°C. The intensity of red colour was measured at 505nm. Inhibition of α glucosidase quantitatively decreased glucose formed which reduced the intensity of the red colour produced.
Screening for in-vitro alpha amylase inhibitory activity
In-vitro porcine pancreatic α amylase inhibitory activity was determined by a method using Chloro-4-nitrophenol α -D-maltotrioside(CNP-G3) as substrate[11]. Pancreatic alpha amylase hydrolyses 2-chloro-4-nitrophenol α -D-maltotrioside(CNP-G3) by cleaving the terminal glucose, to release 2-chloro-4-nitrophenol α -D maltobioside(CNP-G2) which gives a yellow colour whose intensity was measured at 405 nm. Any inhibition reduced the intensity of the yellow colour. For the bioassay, porcine pancreatic α amylase with extract of different concentration/acarbose/vehicle buffer made upto 90µl with phosphate buffer pH 6.9 was taken in wells of microtitre plates and incubated at 370C for 10 min. 125µl of the substrate was added to each well and incubated at 370C for 8 min . The intensity of yellow colour was measured immediately using micro-plate reader.
The % Inhibition(% I) for both α glucosidase and α amylase was calculated using the formula:

The determinations were carried out in triplicates, Average %I was used for determination of IC50.
IC50- the concentration of the test extracts or standard acarbose showing 50% inhibitory activity was determined using Fenny Computer program based on log Probit analysis. The results of the test are recorded in Table 3
Phytochemical studies
Qualitative chemical tests, quantification of starch, saponins, polyphenols, saponin content and flavonoid content was done to evaluate the qualitative and quantitative differences between the mature and tender leaves of M.indica var.. Totapuri . Phytochemical screening of the extracts prepared by refluxation, were subjected to chemical tests. Quantification of total carbohydrate, saponin, polyphenol and flavanoid contents were also done. Starch content was determined by Anthrone method[12], Saponin content was determined by a gravimetric method described by Rajpal et. al[13], Polyphenol content was determined as tannic acid equivalents using a colorimetric oxidation/reduction method[14] that measures all phenolic compounds using Folin Ciocalteau reagent. Flavonoid content of the powdered drugs was determined using aluminium chloride colourimetric method using rutin as standard[15].
Results of qualitative chemical tests are shown in Table 4 starch, saponin, polyphenol and flavanoid contents of mature and tender leaves is represented in Table 5
In the Oral glucose tolerance test, peak blood glucose level after oral glucose loading in both treated and untreated rats was observed at 15minutes(shown in Table 1 ) The peak tapered there afterwards. In extract treated animals however blunting in peak blood glucose level was observed at 15 minutes post glucose loading compared to untreated animals. The blunting of peak was slightly higher with respect to tender leaf extract compared to mature leaf extract. The % inhibition in glucose induced hyperglycemia at different time intervals in treated animals in comparison with untreated animals after oral glucose loading is summarized in Table 2 Tender leaf extract showed significant hypoglycaemic potential during the four hour OGTT period. The same was not true for mature leaf extract. Elevated blood glucose levels was normalised at 4hrs with tender leaf, mature leaf however reduced blood glucose level below normal 4 hrs onwards, post glucose loading.
It was found that both mature as well as tender leaf extracts exhibit alpha glucosidase and alpha amylase inhibitory activity. Mature leaf shows low IC50 value for alpha glucosidase, whereas tender leaf shows low IC50 value for alpha amylase. Both mature and tender leaf extracts also showed oral hypoglycaemic activity in glucose induced hyperglycaemia in rats. Earlier investigations reveal, ethanolic extract of M.indica bark as potent inhibitor of alpha glucosidae and alpha amylase activity in-vitro[16,17]; whereas, cold water extract of mango leaf failed to show any significant inhibition of porcine pancreatic amylase[18]. Our findings reveal that refluxation, and use of aqueous methanol may influence the extraction of principles effective in inhibition of both alpha glucosidase and alpha amylase from mango leaves. Mango leaf extract are reported to increase peripheral utilization of glucose, increase hepatic and muscle glucagon content, promote B cells repair and regeneration and increase C- peptide level. It has antioxidant properties and protects B cells from oxidative stress. It exerts insulin like action by reducing the glycated hemoglobin levels to normal, modulates lipid profile and normalizes STZ induced microalbuminurea. It therefore reduces long term diabetic complications[19]. Our findings strengthen the antidiabetic potential of mango leaf whose mechanism of hypoglycaemic activity may also be due to inhibition of carbohydrate digesting enzymes.
The chemical tests show the presence of phenolic compounds, flavonoids, saponins, carbohydrates and reducing sugars in both tender and mature leaves. Phenolic compounds, saponins and flavonoids are reported for antidiabetic /hypoglycaemic activity[20]. Quantification of starch, saponin, polyphenol and flavonoid contents from the powdered drug material shows higher saponin, polyphenol, flavonoid contents in the tender leaf; however the starch content was higher in mature leaf. Thus phytochemical differences between mature and tender leaf was with respect to the quantity of phytoconstituents only. Leaves of M.indica also are reported to contain mangiferin[4,5], several polyphenols are also identified in the leaves[6]. Oxidative stress plays a key role in insulin resistance and -cell function[20]. Mangiferin and polyphenols exhibit strong anti-oxidant and free radical scavenging activity[20-22]; Besides mangiferin isolated from roots of Salacia reticulate roots inhibits sucrase, isomaltase and rat lens aldose redductase [23]. Thus secondary constituents in mango leaves may mediate hypoglycaemic activity of the leaf extracts and also confer additional benefits of protection against hyperglycaemia induced oxidative stress .
M.indica leaves have been investigated earlier for hypoglycaemic activity[8, 18, 20], however a comparative study of mature and tender leaf is not reported; besides the effect of leaf extracts on carbohydrate digesting enzymes also is not reported. Our findings show hat both mature and tender leaves show hypoglycaemic activity in glucose induced hyperglycaemic rats; the extracts also inhibit rat intestinal alpha glucosidase and porcine pancreatic alpha amylase activities in-vitro. Thus M.indica var. Totapuri mature and tender leaf extracts may be potential agents for normoglycaemic control in patients with genetic risk of diabetes or marginal type II diabetes and/or obesity.