e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Physics, DDE, Annamalai University, Annamalai Nagar, 608 002, Tamil Nadu, India
Received: 22 February 2014 Accepted: 13 March 2015 Published: 18 March 2014
Visit for more related articles at Research & Reviews: Journal of Chemistry
Apparent molar compressibilities () and apparent molar volume () of L-serine, L-asparagine and L-lysine in aqueous and aqueous solutions of cadmium chloride at different concentrations have been determined at the temperature 308.15K from precise density, ultrasonic velocity and time flow measurements using a specific gravity bottle, ultrasonic single frequency interferometer and Ostwald type capillary viscometer respectively. From these data limiting apparent molar compressibilities (), limiting apparent molar volumes (), and their constants (Sk, Sv), viscosity A& B-coefficients and the corresponding transfer parameters (, and ΔB), and hydration number () have been calculated. Transfer parameters have been discussed in terms of ion-solvent and ion-ion interactions in the studied mixtures.
Apparent molar compressibility, apparentmolar volume, Transfer parameter, Viscosity B-coefficient.
Amino acids are the building blocks of all living organisms and incorporate structural features of proteins, their physico-chemical and thermodynamical properties in aqueous solutions are found to provide valuable informations that are important in understanding the stability of proteins. Some of these interactions are found implicated in several biochemical and physiological process in a living cell [1].Measurements of ultrasonic velocity and density values of amino acids in aqueous electrolytes have been of interest with a view to improve the comprehension about the stability native proteins and equilibrium process between “folded” versus“unfolded” forms of proteins [2].Studies on the solubility and stability of proteins have generated a great interest for a long time, but because of complications involved in dealing with these complex molecules, various low molecular weight model compounds are generally taken for investigations [3]. Therefore, physico-chemical properties of amino acids, peptides, and their derivatives which mimic some specific aspects of proteins structure in aqueous solutions have been extensively studied to gainabetterunderstanding of solute-solvent interactions and their role in the conformational stability of proteins [4].
Salts solutions are known to produce remarkable effects on the conformation and properties of proteins [5].The interactions between solvent and various constituent groups of a protein such as the amino acids chain and the peptide backbone group play a central role in the structure, the conformation, and the function of proteins in aqueous solutions [6]. It has been established that metal ions play crucial role in various biological processes. They are generally involved in enzyme regulation, stabilization of structure of reactive molecules, transportations to transmembrane channels and so forth [7]. Cadmium ions are mainly found in the biological systems in the form of Zn/Cd metallothionein (cysteine-rich protein) complex. Metallothionein are the only biomolecules known to normally contain cadmium. Therefore cadmium ion is found in association with proteins whose functions are probably involved with essential trace element (zinc) metabolism [8]. The effect of ions of salt on the solution behaviour of amino acids can be studied through the acoustic volumetric and viscometric properties. Such studies on the solutions behaviour of amino acids are of great relevance because all biological fluids are not pure water and additions have a significant impact on protein stability and functions [9].In this paper we report the densities, ultrasonic velocities, and viscosities ofL-serine, L-asparagine and L-lysine in aqueous and aqueous CdCl2 solutions of (0.5 and 1.0) mol.dm-3 at 308.15K. From these data, various transfer parameters, 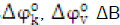 and hydration number nHhave been calculated. These results have been discussed in terms of various interactions operating in these systems.
and hydration number nHhave been calculated. These results have been discussed in terms of various interactions operating in these systems.
Analytical reagent (AR) and spectroscopic reagent (SR) grades which minimum assay of 99.9% of L-serine,L-asparagine, L-lysine and cadmium chloride were obtained from E-Merck, Germany and SD fine chemicals, India, which are used as such without further purification. Doubly distilled degassed water with specific conductance less than 1.29 10-6Ω-1cm-1 was used for the preparation of all solutions. Solutions of cadmium chloride (0.5and1.0 mol.dm-3) were prepared by volume and used on the day they were prepared. Solutions of amino acids in the concentration range of (0 -0.1 mol. dm-3) were made by volume on the molarity concentration scale with a precision of ± × 1x10-4g on an electronic digital balance (Model: SHIMADZU AX -200). The density was determined using a specific gravity bottle by relative measurement method with an accuracy of ± 0.01kgm-3. An ultrasonic interferometer having the frequency of 3 MHz (Mittal Enterprises, New Delhi, Model: F-81) with an overall accuracy of± 0.01% has been used for velocity measurements. An electronically digital operated constant temperature bath (Raaga Industries) has been used to circulate water through the double walled measuring cell made up of steel containing the experimental solution at the desired temperature. The accuracy in the temperature measurement is ± 0.1K. Solution viscosities were measured by Ostwald type capillary Viscometer, which was placed in a water thermostat having temperature stability. Flow time measurements were performed using digital chronometer within ± 0.01s (Model: CASIO HS -10W).The average of at least six readings was used as the final efflux time. The measured viscosity values have an uncertainty of ± 0.001m.Pa.s.
Using the measured data, the following volumetric, compressibility and transport parameter have been calculated using the standard relations.

Molar hydration number has been computed using the relation

Where,βandβoare adiabatic compressibilities of solution and solvent respectively, n1and n2are number of moles of solvent and solute respectively.
The apparent molar compressibility has been calculated from relation,
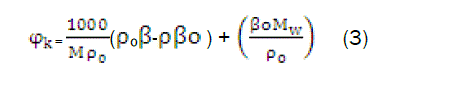
Where,β, and βo,ρ0 are the adiabatic compressibility and density of solution and solvent respectively, Mis the molar concentration of the solute and Mw the molecular weight of the solute. ΨKis the function of Mas obtained byGucker (1993) [10] from Debye Huckel [11] and is given by

where, φ0k is the limiting apparent molar compressibility at infinite dilution and Sk is a constant. φ0k and Sk of equation 4 have been evaluated by least square method.
The apparent molar volume φv has been calculated using the relation
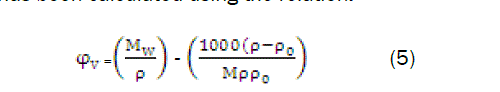
The apparent molar volume φv has been found to differ with concentration according to empirical relation as:

where, φ0v is the limiting apparent molar volume at infinite dilution and Sv is a constant and these values were determined by least square method.
The viscosity A and B coefficients for the amino acids in aqueous cadmium chloride solutions were calculated from the Jones-Dole equation [12].
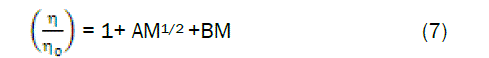
where, η and ηo are the viscosities of the solution and solvent respectively. A is determined by the ionic attraction theory of Falkenhagen –Vernon [13] and therefore also called Falkenhagen coefficient. B or Jones-Dole coefficient is an empirical constant determined by ion-solvent interactions.
Transfer adiabatic compressibility (Δφ0k), transfer volume (Δφ0v)and transfer viscosity coefficient (ΔB) of each amino acid from water to aqueous cadmium chloride solutions have been calculated as:

where, φ0y denotes limiting apparent molar compressibility, limiting apparent molar volume and φ0v viscosity coefficient B.
The experimental values of density (ρ), viscosity (η) and ultrasonic velocity (U) for different molarity composition of each of the three amino acids viz., L-serine, L-asparagine and L-lysine in aqueous and aqueous cadmium chloride solutions (0.5 and 1.0 mol.dm-3) at 308.15K are shown in the Table-1.Further, the values of adiabatic compressibility (β), apparent molar compressibility(φk),apparent molar volume(φv), limiting apparent molar compressibility(φ0k), limiting apparent molar volume(φ0v),and their constants (Sk,Sv), transfer adiabatic compressibility(Δφ0k), transfer volume(Δφ0k), viscosity A&B coefficients and ΔB are shown in Tables 2-3.
Table 3: Values of limiting apparent molar compressibility (φ0k), limiting apparent molar volume (φ0v ) and their constants Sk and Sv, transfer adiabatic compressibility (Δφ0k), transfer volumes (Δφ0v), A and B coefficients of Jones-Dole equation and transfer viscosity coefficient (ΔB) of each amino acids in aqueous cadmium chloride solution at 308.15 K.
In all the three amino acids system thevalues of density (Table-1) increases with increase in molar concentration of amino acids as well as cadmium chloride (CdCl2) content. This increasing trend suggests a moderate strong electrolytic nature in which the solutes(amino acids) tend to attract the solvent (aqueous cadmium chloride) molecules.Further, it is observed from the Table–1that the values of ultrasonic velocity increases with increase in concentration of amino acids but it is found to decreases with increasing the CdCl2 content in all the three systems studied. The factors apparently responsible for such behaviour may be the presence of interactions caused by the proton transfer reactions of amino acids in water and cadmium chloride mixtures.Molecular interaction is thusresponsible for the observed increase in density and ultrasonic velocity in these mixtures. The increase in ultrasonic velocity in these mixtures may be attributed to the cohesion brought about by the ionic hydration.
In all the three systems the value of adiabatic compressibility (Table2) decreases with increase in concentration of amino acids as well as CdCl2. The decrease in adiabatic compressibility is attributed to the influence of the electrostatic field of ions on the surrounding solvent molecules (Cd2+, Cl-) so called electrostriction.The magnitude of β values is larger in L-serine than other two amino acids. The larger β value which shows molecular associations/interactions is greater in L-serine than that of other two amino acids. Amino acid molecules in the neutral solution exist in the dipolar form and thus have stronger interaction with the surrounding water molecules. The increasing electrostrictive compression of water around the molecules results in a large decrease in the compressibility of the solutions [14].The interaction between the solute and water molecules in the solvent is termed hydration.From the Table-2 it is observed that the values of nH are positive in all the systems studied and such positive values of indicate an appreciable solvation of solutes.These values are found to decrease with increasing the content of amino acids as well as cadmium chloride in all the three systems studied. The decreasing values of nH which indicate the increase in solute-co-solute interaction. The decreasing behaviour of nH shows that cadmium chloride has a dehydration effect on the amino acids.
The negative values φk of and φvTable-2 are increases with increase in concentration of amino acids as well as CdCl2. The negative values of φk and φv in all systems indicate the presence of ion-solvent interactions.The limiting apparent molar compressibility φ0k provides information regarding ion-solvent interactions and Sk, that of ion-ion interactions in the solution. From the Table-3 it is observed that φ0k values are negative and it increases with increasing the concentration of cadmium chloride in all the systems studied. Appreciable negative values of φ0k and its behaviour for all the systems reinforce our earlier view that existence of ion-solvent interaction in the mixtures. The magnitude of φ0k is in the order L-serine >L-asparagine > L-lysine. The valuesof Sk exhibits positive value and it decreases with increasing the concentration of cadmium chloride in all the three amino acids. The positive values of Sksuggest that ion-ion interactions are relatively stronger. It is well known that solutes causing electrostriction lead to decrease in the compressibility of the solution. This is reflectedby the negative values of Ψk of the amino acids.
The volume behaviour of a solute at infinite dilution is satisfactorily represented by φ0v which is independent of the ion-ion interactions and provides information concerning ion-solvent interactions.Table-3 reveals that the negative values of φ0v increases with the addition of cadmium chloride contents in all the systems studied. The increase in φ0vmay be attributed to the increased hydrophilicity polar character of the side chain of the amino acids. The magnitude of φ0v is in the order of L-serine >L-asparagine >L-lysine.
It is evident from the Table-3 that the positive Sv indicates the presence of strong ion-ion interactions and less complex ion formation taking place in the ternary mixtures [15]. The negative Sv values are associated with hydrophobic solutes [16].Generally, the interactions between amino acids and cadmium chloride can be classified into:(i) Ion-charged group interactions occurring between Zwitter ions(NH3+ and COO-) of amino acids and cations (Cd2+) and anions (Cl-) of CdCl2 and (ii) Ion-non polar group interactions occurring between ions of cadmium chloride (Cd2+, Cl-) and the hydrophobic side chain of amino acids.The Δφ0k and Δφ0v andvalues can also be explained on the basis of co-sphere overlap model [17] in terms of solute-cosolute interactions. According to this model, ion-charged group interactions contribute positively, whereas ion-non polarinteractions contribute negatively to the Δφ0k and Δφ0v values. Therefore, the presently observed positive Δφ0k and Δφ0v (Figures1&2)values in the presence of CdCl2 on the whole indicate that ion-charged group interactions dominateoverthe ion-nonpolar groupinteractions in these solutions.
The Δφ0v values increase in the order L-serine >L-asparagine > L-lysine at all concentration of CdCl2. The positive Δφ0v values may be attributed to a decrease in the shrinkage volume in the presence of CdCl2 solutions. This may be due to stronger interactions between amino acids and ions of co-solute
A perusal of Table-3 shows that in all the three systems the values of A and B coefficients are positive and these values are increases with increasing the concentration of CdCl2. Since A is a measure of ionic interaction, it is evident that the positive A-coefficient has strong ion-ion interaction whereas negative A-coefficient has weak ion-ion interactions in the mixtures studied [18].The viscosity B-coefficient reflects the effect of ion-solvent interactions on the solution viscosity. The positive B values suggest the presence of strong ion-solvent interaction. Further, ΔB values show in Figure 3 which supports the result obtained from Δφ0k and Δφ0v discussed above. The magnitude of ΔB is in the order L-serine >L-asparagine >L-lysine.
In the summary, limiting apparent molar adiabatic compressibility φ0k,limiting apparent molar volume φ0v viscosity B-coefficients ofL-serine, L-asparagine and L-lysine in aqueous solutions of CdCl2 have been determined at 308.15 K. The various transfer values ( Δφ0k and Δφ0v and ΔB ) are positive in all cases and decreases with increases in the concentration of CdCl2. From the magnitude of φ0k, Δφ0k and ΔB it can be concluded that L-serine possess strong ion-solvent inter actionsthan the other two amino acids. The transfer properties suggest that ion-charged group interactions are dominating over ion-non polar group interactions.