ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Gopinath S.M, Vidya G.M*, Dayananda.K.S, Narasimha Murthy.T.P, Ashalatha.
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Extracts of two ethnobotanically selected medicinal plants used in the Isolation and characterization of bioactive compounds which are investigated against anti-HIV properties. Enzymes and proteins that play a role in the HIV life cycle in which antiviral activity was studied through the pepsin-based assay targeting the HIV promoter activation induced by either the HIV-1 Tat protein. Assay methods to determine antiviral activity include multiple-arm trials, randomized crossover studies. These plants are used to investigate the bioactive compounds like tannins, saponins, flavanoids, coumarins, alkaloids, terpenoids and phenolic compounds against anti-HIV activity.
Keywords |
| Anti-HIV, Bio-active compounds, Ethnobotanical, Pepsin assay, Antiviral |
I. INTRODUCTION |
| Andrographis paniculata is an annual herbaceous plant in the family Acanthaceae, native to India and Sri Lanka. It is widely cultivated in Southern and Southeastern Asia, where it has been traditionally used to treat infections and some diseases. Mostly the leaves and roots were used for medicinal purposes. A.peniculata is used in traditional Siddha and Ayurvedic systems of medicine as well as in tribal medicine in India and some other countries for multiple clinical applications.The herb has a number of purported medicinal uses, although research has found evidence of its effectiveness is limited to treatment of upper respiratory infection, ulcerative colitis and rheumatic symptoms; in particular, there is no evidence of its effectiveness in cancer treatment[1]. The aerial parts of the plant (Leaves and stems) are used to extract the active phytochemicals. The leaves contain the highest amount of the andrographide, the most medicinally active phytochemicals in the plant, while the seeds contain the lowest. The primary medicinal compound of Andrographis is andrographide[2]. |
| AndrographispaniculatacommonlyknownasKingofbittersbelongstothefamilyAcanthaceae.It hasbeenusedforcenturiesasamedicinalherbforthetreatmentofuppergastrointestinaltractandupperrespiratoryinfections,fever,her pesandotherchronic diseases.Ithasabroadrangeofpharmacologicaleffects.TheprimarymedicinalcomponentofA.paniculatais andrographolide,whichisaditerpenelactone.Andrographolidehasbeenreportedforitsanti-cancer,anti- HIV,cardioprotectiveandhepatoprotectivepropertiesamongothers[3].Cinnamomumverum also called as true cinnamon, Ceylon cinnamon or Sri Lanka cinnamon, it is a small evergreen tree belonging to the family Lauraceae, native to Sri Lanka. |
| Among other spices, its inner bark is used to make cinnamon.Cinnamon verum gives numerous beneficial health effects against the HIV activity, and this plant exhibits anti-inflammatory property, anti-microbial activity, reducing cardiovascular disease, boosting cognitive function and reducing risk of colonic cancer.Recent studies suggest that consumption of cinnamon on a daily basis could significantly lower blood sugar and cholesterol levels and making it extremely helpful for patients Suffering from Type 2 diabetes. Cinnamon essential oil has significant antioxidant and antimicrobial properties as well [4]. |
| Plants and other natural products present a large repertoire from which to isolate novel anti-HIV active compounds. HIV-1 is the cause of the world epidemic and is most commonly referred as HIV. It is a highly variable virus, which mutates readily. There are many different strains of HIV-1, which can be classified according to groups and subtypes; there are two groups, M and O. Within group M, there are currently known to be at least ten genetically distinct subtypes of HIV-1. These are subtypes A to J. In addition, Group O contains another distinct group of heterogeneous viruses [5].Treatment of HIV infected patients with currently available highly active anti-retroviral (HAART) drugs, though successful in reducing the burden of the disease but is associated with various side effects, including the emergence of drug resistant HIV strains. Hence, it is imperative to discover novel anti-HIV agents from natural sources that may have lesser side effects. Various studies have shown anti- HIV properties of the extracts prepared from a variety of plants. The plant extracts or purified phytochemicals may exhibit anti-HIV activity by inhibiting virus entry/fusion, HIV-1 reverse transcriptase (RT), protease or its integrase activity [6]. |
II. REVIEW OF LITERATURE |
| Paniculata has been reported as having antibacterial, antimalarial, antifungal, antiviral, antiinflamatory, fertility effects and protection of the liver and gallbladder. And also in broad range of pharmacological effects. The main activity of this plant is that it willpufifiesa bloodwhen it is entered into the human body, so that it cures Leprosy, Gonorrhea, Seasonal fevers, and also helpsin the prevention and treatment of the common cold. And it is extremely used as a hepatoprotective agent in Indian system of medicine [7]. |
| HIV begins its infection of a susceptible host cell by binding to the CD4 receptor on the host cell. CD4 is present on the surface of many lymphocytes, which are a critical part of the body’s immune system. It is now known that a co-receptor is needed for HIV to enter the cell. Several reviews on the natural products for the chemotherapy of HIV infection have been published earlier [8]. As a part of our screening program to investigate antiviral activity from plants [9]. The importance of investing in the high growth sectors of biotechnology and phytomedicine was also articulated in the founding document of the New Partnership for Africa’s Development (NEPAD), and adopted by the African Biosciences Initiative (NEPAD, 2001; African Biosciences Initiative, 2005) [10]. |
III. MATERIALS AND METHODS |
| Detection of anti-HIV activity of the extracted compound |
| Enzyme pepsin inhibition assay |
| Pepsin has a quite close resemblance in proteolytic activity with HIV-1 protease protease one key enzyme of HIV-1 life cycle as both of them belong to an aspartate enzyme family. This enzyme was used as a substitute of HIV-1 protease to check out anti-HIV activity of plant extracts in the present investigation. |
| Preparation of Hemoglobin |
| Hemoglobin was prepared as 2.5 gm hemoglobin powder (HiMedia) was dissolved in 100 ml distilled water. It was blended, at maximum speed for 5 min and then filtered through gauze. Eighty ml of filtrate was diluted with 20 ml of 0.3N HCL and stored in 4º C until further use. |
| Pepsin assay |
| Pepsin assay was carried out as described by Singh et al., Briefly, 50 μg pepsin (HiMedia), 800 μg hemoglobin (HiMedia) and different concentrations of each extract were taken in 500 μl of reaction mixture. The mixture was allowed to incubate at 37º C for 20 min. After incubation, 700 μl of 5% trichloro acetic acid (TCA) (HiMedia) was added to stop the reaction. It was then centrifuged (Rotina 38R) at 14000 rpm for 5 min and the supernatant was collected. Optical density was recorded spectrophotometrically (Cary 50 Bio UV-Visible spectrophotometer) at 280nm. Pepstatin-A (Sigma) was included as a standard. Negative control without extract(s) was set up in parallel. Separate blanks were used for extracts. All the determinations were done in triplicate. Percent Inhibition was calculated as, Inhibition (%) = (A negative control-Atest) /A negative control X 100. Was Aabsorbent. The result is expressed as ICâÃâÃâ¦Ã¢ÃâÃâ¬. ICâÃâÃâ¦Ã¢ÃâÃ⬠values were calculated using a Microsoft Excel program. |
| Biophysical characterization of plant extract |
| Thin layer chromatography |
| TLC for the identification of anti-HIV activity in crude extracts |
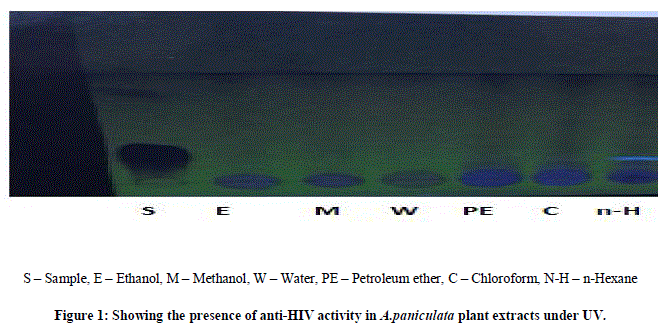
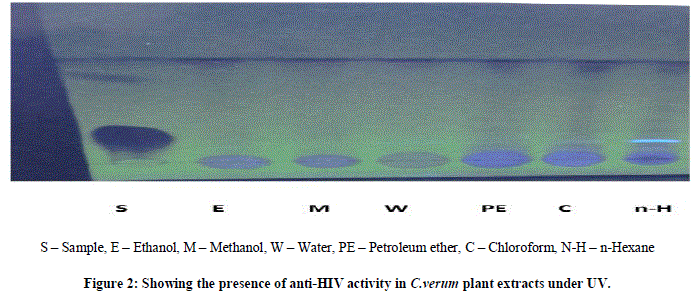
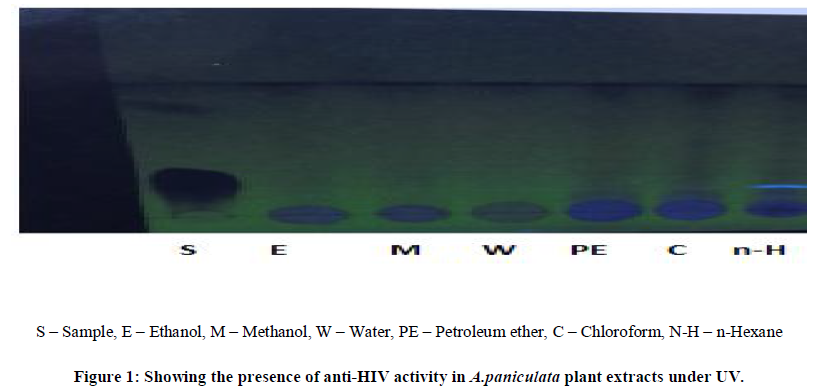
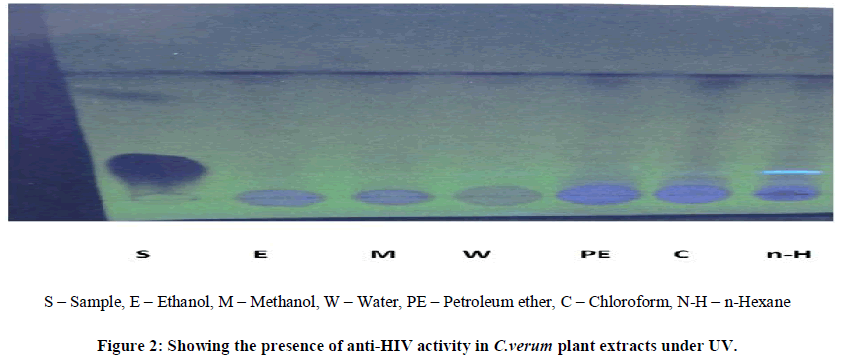
| Thin layer chromatography (TLC) was employed in this study to analyze the presence of anti-HIV activity in the A.paniculata and C.verum crude plant extracts of the different solvents i.e., ethanol, methanol, water, petroleum ether, chloroform, n-hexane at different temperature for 5 min followed by one or two cycles.Normal phase silica gel GF precoated TLC (scored 10×20 cm) plates; 250 microns were used. The solvent extracts were applied as separate spots to a TLC plate about 1.3 cm from the edge (spotting line), using micro tips. |
| The mobile phase, chloroform/methanol=9: 0.6 (v/v), were used for each crude extract. All TLC separations were performed at room temperature, i.e. 18-23ºC. After sample application the plates were placed vertically into a solvent vapor saturated TLC chamber. The spotting line was about 0.5 cm from the developing solution. After the mobile phase had moved about 80% from the spotting line, the plate was removed from the developing chamber.Indeed, anti-HIV activity presence has been proven by several fluorescent green stains under UV to 366nm. The sheets were dried in hot air oven for 4-5 min, the colorization was developed by passing iodine fumes on dried sheets. RF values were tabulated and compared it with standard RF values. |
| TLC for the identification of anti-HIV activity in fractions |
| Normal phase silica gel GF precoated TLC (scored 10×20 cm) plates; 250 microns (Analtech, Uniplate No. 02521) were used. The fractions collected were applied as separate spots to a TLC plate about 1.3 cm from the edge (spotting line), using micro tips.The mobile phase, chloroform/methanol=9: 0.6 (v/v), were used for each fraction. All TLC separations were performed at room temperature, i.e. 18-23ºC. After sample application the plates were placed vertically into a solvent vapor saturated TLC chamber. The spotting line was about 0.5 cm from the developing solution. After the mobile phase had moved about 80% from the spotting line, the plate was removed from the developing chamber. |
| The sheets were dried in hot air oven for 4-5 min, the colorization was developed by passing iodine fumes on dried sheets. RF values were tabulated and compared it with standard RF values. |
IV.RESULTS AND DISCUSSION |
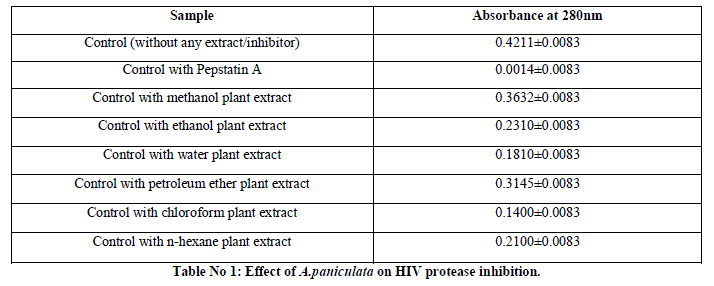
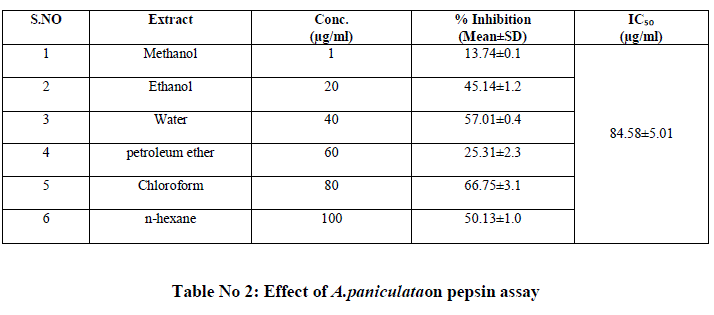
| The two plant extracts showed a very significant inhibition of pepsin enzymatic activity Table No 1 and 2 may be these extracts inhibit the activity of HIV protease. Many similar works have been made with plant extracts. Graph 1 and Graph 2 Shows nonlinear regression dose response plot determining ICâÃâÃâ¦Ã¢ÃâÃ⬠values of A.paniculata and C.verum plant extracts. Both the plant extracts showed potent inhibitory activity with ICâÃâÃâ¦Ã¢ÃâÃ⬠values of 84.58 and 56.08 μg/ml, respectively as shown in Table No 2 and Table No 4. This shows the inhibitory activity of pepsin enzyme should also inhibit activity of HIV protease. The inhibitory activity could be attributed to high coumarins and flavonoids content. The inhibitory effect was confirmed by the IC50 values. |
 |
 |
| Y=bottom+ (top-bottom) / (1+10^ ((logEC50-X)), |
| Where X is the logarithm of concentration and Y is the response. Y increases in a sigmoid shape. |
 |
 |
| Where X is the logarithm of concentration and Y is the response. Y increases in a sigmoid shape. |
 |
 |
IV. CONCLUSION |
| Medicinal plants have a long history of use of treating many diseases in many developing and developed countries. So herbal medicines can be developed as a safe, effective and economical alternative for AIDS. This work presents evidences that A. paniculata and C. verum plants contain anti-HIV bioactive compounds that could be developed into newer drugs to manage HIV/AIDS. This evidence should persuade further research. |
References |
|