ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K. Diouri1, A. Kherbeche2, A. Chaqroune1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The aim of this present work is evaluation of the effect of marble powder, which is an industrial discharge, in the adsorption of “Congo Red” dye, used in traditional industries of textile. The effect of various experimental factors; adsorbent dose, contact time (equilibrium is established after 90 minutes), dye concentration and pH media, were studied by using the batch technique. The isotherms of adsorption data were analyzed by various adsorption isotherm models such as Langmuir and Freundlich. The dye Congo Red adsorption by marble powder made have excellent performance related to the adsorption potential.
Keywords |
| Adsorption isotherm, Marble powder, Congo Red. |
INTRODUCTION |
| Azo dyes are the most important and most used for coloring in the textile industries, endanger human health due to either toxic or mutagenic and carcinogenic effects [1] .They are very stable because of their aromatic structures, are difficult to degrade. They can cause environmental pollution problems by the formation of hazardous aromatic amines through metabolic processes in plants and animals. They can cause carcinogenic and mutagenic effect to animals, even at low concentrations [2] .The azo dye can also cause coloration of the surface of the water, which would block the penetration of sunlight and lower oxygen levels in the water. Moreover, the azo dyes are difficult to deteriorate owing to their complexity aromatic and their molecular structure which is stable [3]. |
| Congo red (sodium salt of benzidinediazo bis-1-naphthylamine-4-sulfonic acid) is an azo dye and the first synthetic anionic dye of azo dyes, which is synthesized by coupling of the tetra benzidine with two molecules napthionique acid [4]. Congo red (sodium salt of benzidinediazo bis-1-naphthylamine-4-sulfonic acid) is an azo dye and the first synthetic anionic dye of azo dyes, which is synthesized by coupling of the tetra benzidine with two molecules napthionique acid [4]. |
| This dye can cause allergic reactions [5] .Congo Red Treatment in wastewater is difficult because the dye is generally present as sodium salt which gives it very good solubility in water. Through their chemical structures, dyes resist fading when exposed to light, in water and in contact with many chemicals product, they are difficult to be discolored when they are released into the aquatic environment. |
| Many processing techniques including biological treatment, coagulation, flotation, oxidation, ozonation and nanofiltration are used for the removal of dyes in wastewater [6-8]. |
| Adsorption is recognized as one of the most efficient methods for removing dyes from aqueous solutions. In recent years, compounds such as the nanoporous silica, hematite, γ-Fe2O3 / SiO2, CuFe2O3, mesoporous TiO2, bentonite, activated carbon ... were used to eliminate the azo dyes [9-11]. |
MATERIALS AND METHODS |
| Adsorbate |
| Concentrations of dyes were determined by finding out the absorbance characteristic wavelength using UV spectrophotometer (Jasco). A standard solution of the dye was taken and the absorbance was determined at different wavelengths to obtain a plot of absorbance versus wavelength. The wavelength corresponding to maximum absorbance (lmax) was determined from this plot. The lmax for CR are found to be 497 nm. Calibration curves were plotted between absorbance and concentration of the dye solution. |
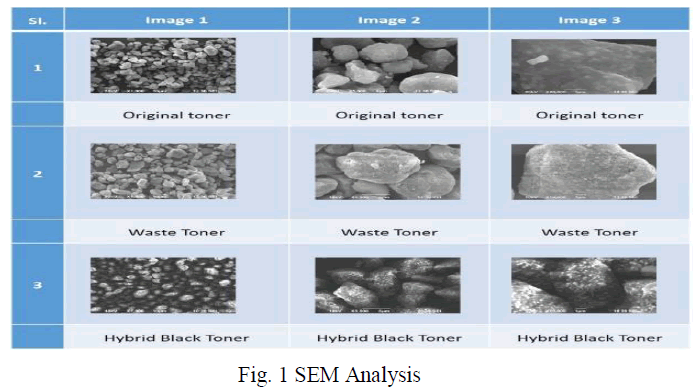 |
 |
 |
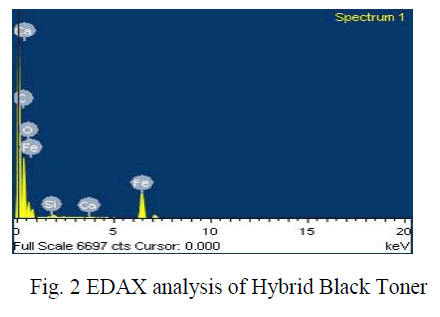
 |
 |
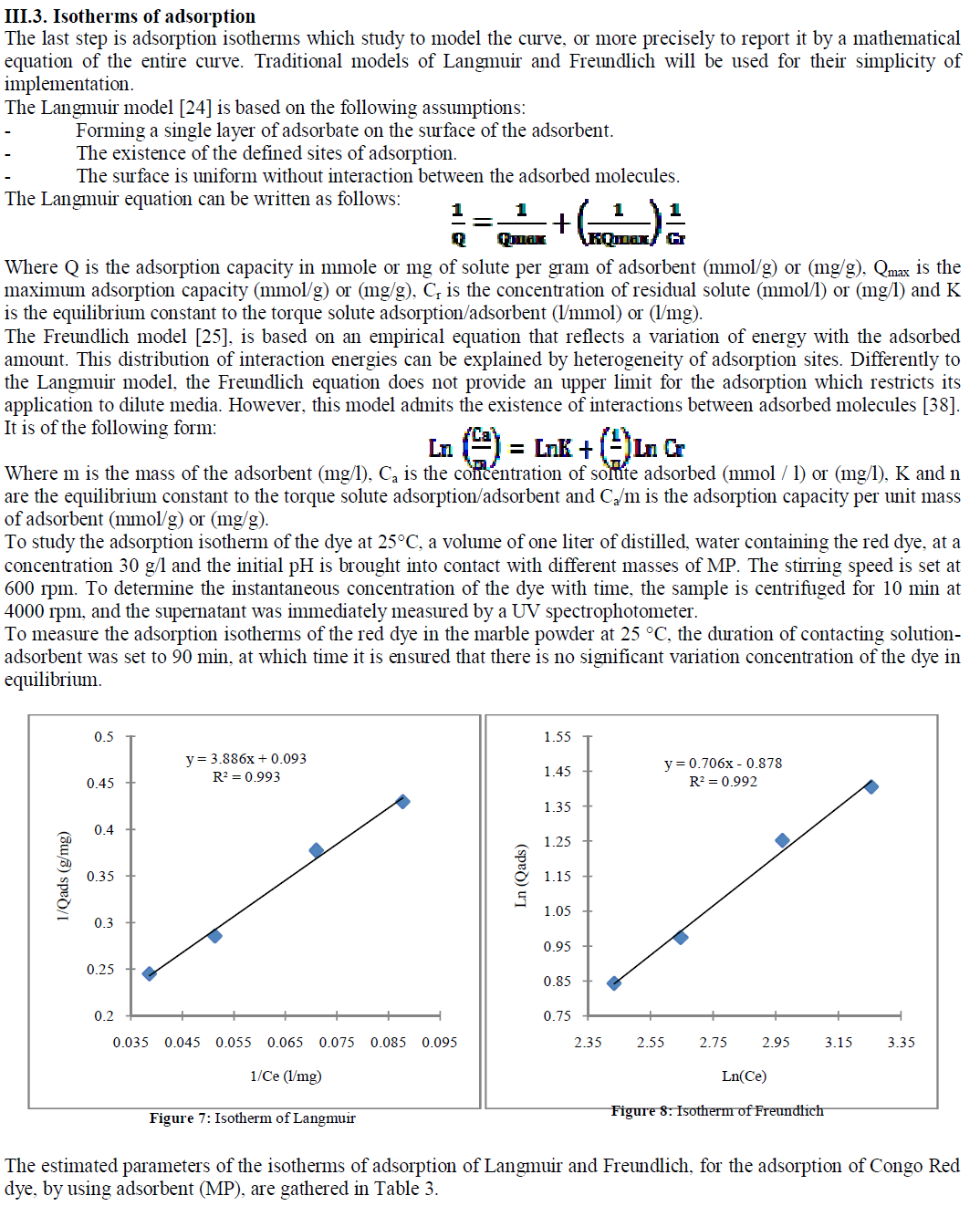 |
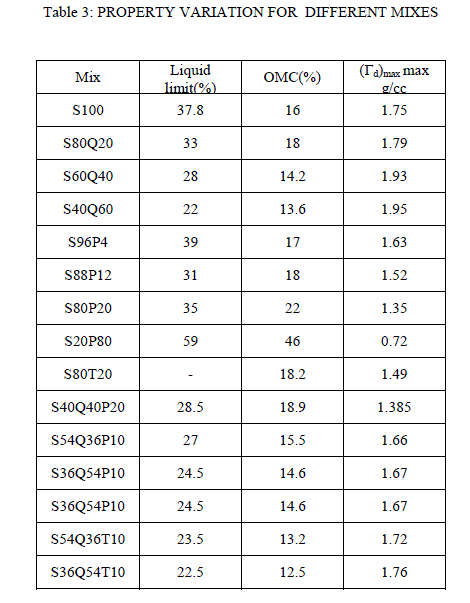 |
| Table 3 : The estimated parameters of the isotherms of adsorption of Langmuir and Freundlich |
CONCLUSION |
| Marble powder has shown its effectiveness in removal of the dye in aqueous solution. The adsorption is highly dependent on various parameters such as the mass of adsorbent, contact time, pH and initial concentration of the dye. The reduction rate of the Congo Red has increased with the increase of the mass of the adsorbent. On the contrary, the rate of reduction decreased with increasing concentration of the dye. While the change in pH has little influence on the adsorption of CR dye. |
| The kinetics of dye adsorption on the marble powder is not fast, the equilibrium is reached after 90 min. The dye adsorption isotherms by the marble powder following the model of Langmuir and Freundlich. |
References |
|