ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
G.Mathubala 1, Dr.V.Krishnasamy1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
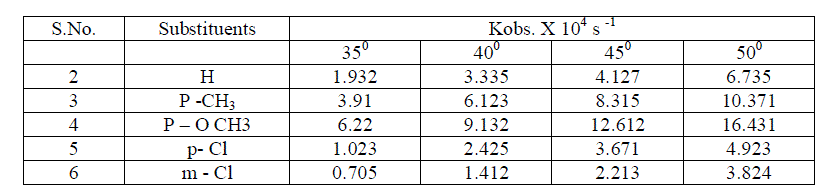
The kinetic study of simple and substituted anils ware studied under various conditions viz., solvent ,oxidants , electrolyte , catalysts and mixture of catalysts and temperature; simple and substituted anils , pyridinium Chloro Chromate(PCC) ,Pyridinium Dichromate ( PDC) were prepared in the laboratory and kinetic study were carried out using Pyridinium dichromate , substrate , perchloric acid and osmium tetraoxide . The graphs were drawn at all relevant places and results were obtained in full satisfaction
Keywords |
| diverse nature , chloramine [CAT], sulphonamide , oxidants , water – bath , evaporating , stirring |
INTRODUCTION |
| Oxidation of Anils Among oxo derivatives of variable valence metals , Chromium compound play the most important role , in oxidative reaction .A number of Chromium reagents are readily available . Almost every oxidisable fuctional group may undergo Chromium oxidation .Chromium ( VI ) containing reagents include Chromium acid , dichromate ion , Chromyl chloride , chromyl acetate , t – butyl Chromate , Chromylnitrate and Co – ordination complexes of Chromium trioxide .1-2 Chromium ( VI oxidations are usually performed under acidic condition . Co – solvents like ( Jone’s reagent ) benzene , methylenechloride ( Two Phase system ) are often added in order to deal with water insoluble organic complexes . The oxidation rate is generally high under acid catalysed conditions , however the low pH of the reaction medium and the presence of water favouring hydrolytic condition exclude the use of this class of reagents for the oxidation of molecules containing acid sensitive groups. Polymer supported Chromium ( VI) reagents have also been developed. These reagents offer the advantage of reducing the work of procedure to more filtration . Recently some neutral or almost neutral Chromium (VI) reagents have been developed to effect oxidation under mild conditions. Pyridinium Chlorochromate (PCC) introduced by Covey et al3 is widely used in the oxidation of alcohols. Pyridinium flourochromate has a less pronounced acidity and is an effective agent for the oxidation of Plycyclic organic substitutes . PFC was developed by Bhatachargee and coworker . 4 In 1986 , Narayanan and Balasuramaniam5 introduced Pyridinium bromochromate . This is an efficient oxidant for alcohols and a brominating agent as well .Firouzahadi and Coworkers used Zinc chromates. This is a very useful reagent for the oxidation of variety of organic compounds , including alcohols , oximes , olefins and aromatic hydrocarbons. |
| They have also shown chromium peroxide complexes as versatile , mild and efficient oxidants in organic synthesis.Chlorotrimethyl Silone , Imidazolium dichromate , 2,2’ – Bipyridyl chlorochromate (BPCC) and the corresponding chromate (BPC) have been proposed as oxidants for the oxidation of the hydroxyl group to thr carbonyl group.4 – ( dimethyl amino ) – pyridinium chlorochromate and chromyl nitrate have been shown to be efficient and mild oxidant in aprotic media.Naphthyridium chlorochromate , Pyrazinium chlorochromate , tetrabutyl ammonium chlorochromate , dimethylpyrazole chromate , tetrabutyl ammonium dichromate , Pyridine complex of oxodiperoxochromium and the dipyridine complex of chromium trioxide are some of the Chromium (VI) oxidants introduced in the last twenty years.Quinolinium Chlorochromate was used by Bhavani et al to oxidize methyl phenyl sulphoxides.Quinolinium dichromate was introduced by Balasubramanian and Prathiba 6 as an effective oxidant under non – aqueous conditions. Pyridium Chlorochromate (PCC) is irreplaceable in the generation of functional groups in highly unsaturated carbinols and pyrols . yet mildly acidic character of Pyridinium chlorochromate precludes its use with acid sensitive substrates or products. Pyridium Chlorochromate oxidation has been investigated under various condition on different substrates .Despite the introduction of numerous oxidants based on chromium (VI) not much work has been done to investigate the mechanism of oxidation in these Several oxidation of anils have been studied : |
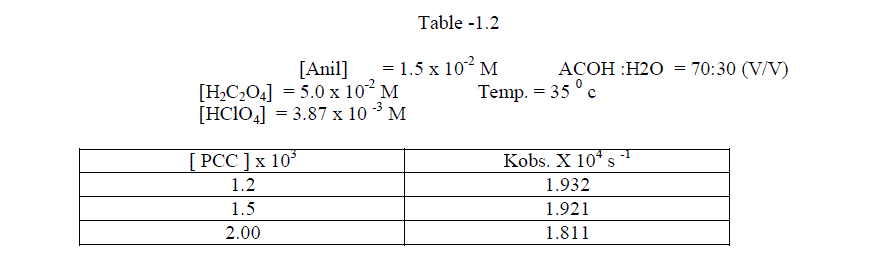
1.2.1.Effect of varying PCC concentration |
| The reaction was investigated by varying the concentration at constant substrate concentration .The reaction was found to be first order with respect to PCC [ Table – 1.2 ] |
 |
1.2.2.Effect of varying the concentration of Anil |
| The reaction was carried out under pseudo – first order conditions . In this study , the concentration of PCC , Oxalic acid , Perchloric acid and percentage of oxalic acid were kept constant and the concentration of the alone varied . The reaction was found to be first order with respect to anil . [ Table – 1.3 ] |
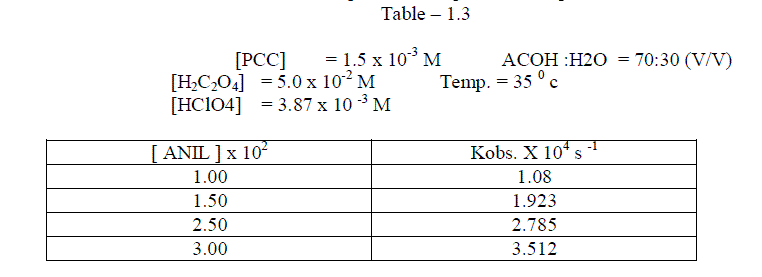 |
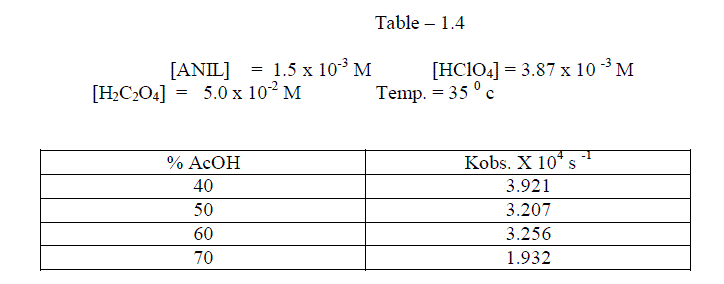
1.2.3.Effect of varying solvent Composition |
| The reaction was studied by the different composition of acetic acid under constant [Reactants ]. It was found that as the percentage of acetic acid increased , the rate decreased. [ Table – 1.4 ] |
 |
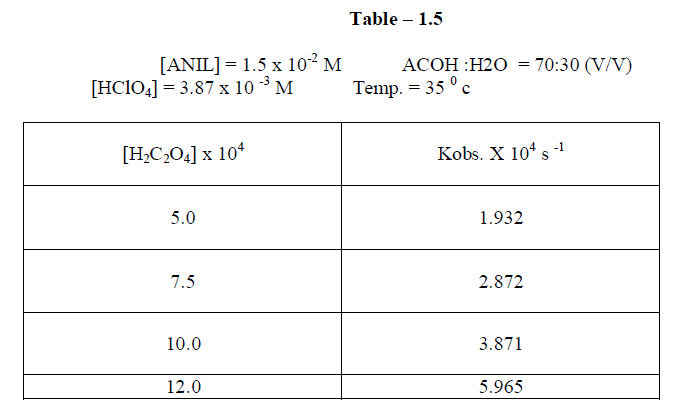
1.2.4Effect of varying Oxalic acid |
| The reaction was carried out under by varying , the concentration of , Oxalic acid and by keeping other [reactans] constant. . The result indicate that as the concentration of oxalic acid increased , the rate constant also increased. |
 |
1.2.5.Effect of varying Perchloric acid |
| The reaction was studied by varying , the concentration of perchloric acid and by keeping other [reactans] constant. . The result indicate that as the concentration of Perchloric acid increased , the rate constant also increased. [ Table –1.6 ] |
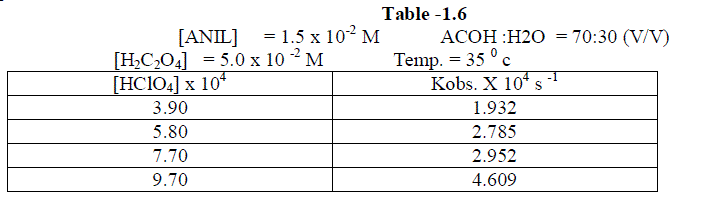 |
1.2.6.Effect of varying Sodium Perchlorate |
| To follow the Primary Salt effect , the reaction was studied with varying concentration of Sodium Perchlorate by keeping other [reactants] constant. It was found that as the concentration of Sodium Perchlorate increased , the rate constant also increased. This shows that the participation of ions which are similar in their sign or dipole –ion interaction in the rate determining step. [ Table 1.7 ] |
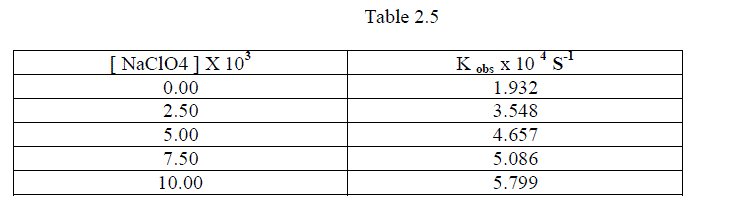 |
1.2.7.Effect of MnSO4 |
| The reaction rate decreases tremendously with the increase in the concentration of MnSO4 [ Table - 1.8 ] This may be due to the formation of Cr(VI) in the rate determining step. |
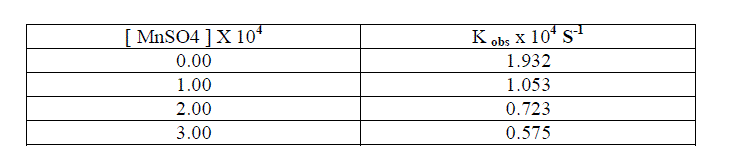 |
1.2.8 Effect of temperature |
| Effect of temperature The rate of oxidation of some meta and para substituted anils had been studied at four different temperatures viz., 350 c , 400 c, 450 c and 50 0 c . It was observed , as we expected ,that the rate increases very much with increase of temperature . [ Table – 1.9 ] |
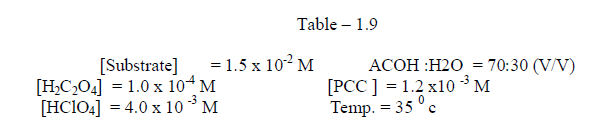 |
 |
II.KINETICS OF CHLORAMINOMETRIC OXIDATION |
| Coull and co –workers were thr first to investigate the kinetics and mechanism of decomposition of H2O2 by CAT in the presence of HCl.The reaction was found to be first orders each of [ H2O2] and [CAT [ , but inverse first order dependence on the concentration of RNH2. Oxidation of kinetics of Alpha – hydroxyl acids1 with CAT in alikaline medium in the presence of Os (VIII)as catalyst was found a follow first order each in [ CAT] AND Os (VIII ) and was independent of substrate concentrations . Os (VIII ) catalysed oxidation of acetone and ethyl methyl ketone by CAT was reported3 . |
III.EXPERIMENTAL |
3.1.Preparation of Anils |
| General method of preparation of Anils |
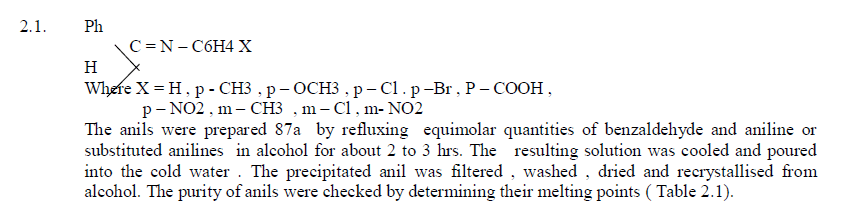 |
3.2.Preparation of Chloramine – T (CAT) |
| a) Preparation of Pure p- tolenesulphonamide The procedure used was that of Vogel.79 Ten grams of p- tolenesulphonylchloride and 20 g of ammonium carbonate were ground in a mortar to a fine powder. The mixture was heated in a an evaporating dish on a water bath for 1 – 2 hrs. and the mixture was stirred frequently with glass rod. After cooling the mixture was extracted with a little cold water to remove excess ammonium salts . The crude p- tolenesulphonylchloride was recrystallised from boiling water and dried at 100 0 C . The pure product melted at 1380 C. |
| b) Preparation of Dichloramine – T ( DCT ) This was prepared by the method of Krausand crede . p- tolenesulphonamide was dissolved in ten parts of 1 : 10 caustic soda and diluted with twenty parts of water . Chlorine gas was then passed into the solution until a voluminous white precipitate of dichloramine – T was formed .This was collected on a Buckner funnel , thoroughly washed twice with 5 – 8 parts of water and finally with enough 10% alcohol to make a thin paste . The dilute alcohol washing was done very quickly and the substance was separated with air of a vacuum filter . It was then dried in a vacuum drier dichloramine –T was obtained , and its melting point is 82 - 830 c. |
| C ) Preparation of Chloramine – T (CAT) Forty five ml of 10% sodium hydroxide solution was heated to a temperature of about 800 and to which added 6 g of dichloramine – T in small quantities , strring the mixture gently after each addition until a clear solution was obtained. When the addition was completed , the hot solution was filtered and then allowed to cool spontaneously . The crystals were filtered with suction , washed with little brine solution , and dried in a desiccators over anhydrous calcium chloride . It was recrystallised from water ; m.p. 175 – 179 0 C. |
3.3.Reagents |
| Analar samples are sodium perchlorate ,Sulphuric acid , Perchloric acid , Sodium chloride , Osmium tetroxide were used. Ruthenium chloride was dissolved in water containing dil. HCl and the solution was standardized by the method of Houriuchi and Ichijo13 |
IV.KINETIC MEASUMENTS |
| The reaction was carried out in 60 % aqueous acid (V/V) . Following solutions of desired concentrations were prepared . |
| 1. Anil in Acetic acid |
| 2. Chloramine – T in water |
| 3. Sodium perchlorate in water |
| 4. Sodium Chloride in water |
| 5. Ruthenium chloride in water containing dilHCl |
| 6. Osmium tetroxide in dil.Sodium hydroxide . |
V.STOICHIOMETRY |
| The stoichiometry of the reaction was determined by studying the reaction under the conditions of CAT > > anil and it was found to be 1:1 |
VI.RESULTS AND DISCUSSINS |
6.1.Effect of varying Os ( VIII ) |
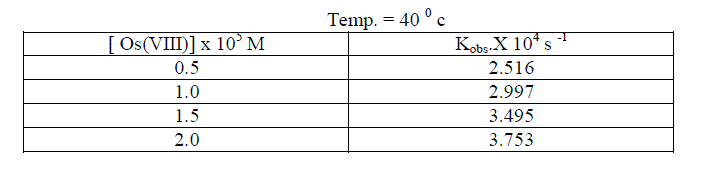
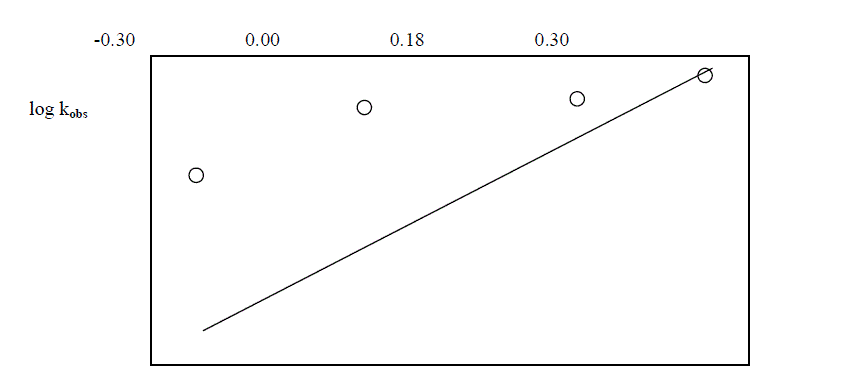
| The reaction was carried out by varying the concentration of Os (VIII) and by keeping othe [ reactants ] constant. The results indicate that as the concentration of Os ( VIII) increased , the rate constant also increased . the plot of log Os (VIII ) Vsa log kobs. Gave a straight line of slope 0.29 [ Table 5.4. Fig.2] |
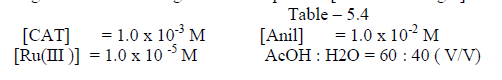 |
 |
 |
6.2.Effect of varying Ru(III) concentration |
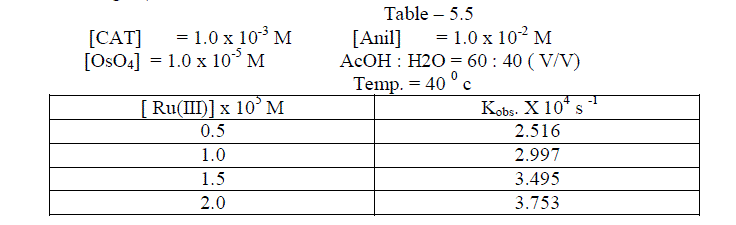
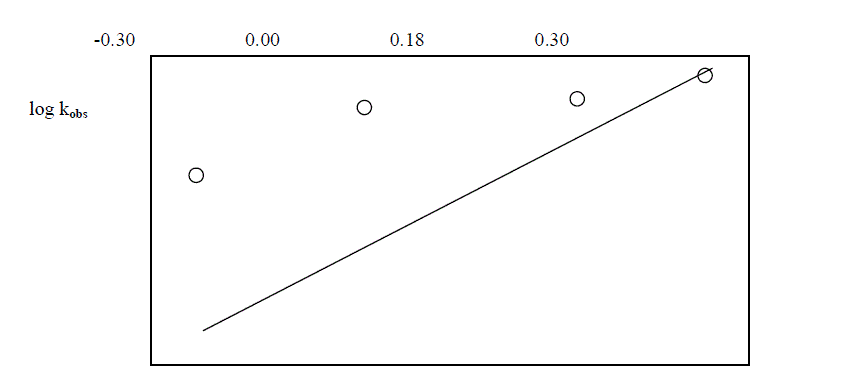
| The reaction was followed with varying concentration of Ruthenium trichloride and by keeping other [reactants] constant. It was found that as the concentration of Ruthenium trichloride increased , the rate constant also increased . The plot of log [ Ru(III)] against logKobs. gave a straight line of slope of 0.34 ( Table 5.5 ; Fig. 5 ) |
 |
 |
References |
|