ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sayana K R1, P.Indira2, K. V. R. Murthy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present paper reports the photoluminescence (PL) of rare earth ions (RE-Eu,Ce and Eu,Ce,Ga) doped SrS phosphor. The phosphors were synthesized by standard solid-state reaction method under carbon reduction atmosphere and the starting materials are grounded by using mortar and pestle, and these materials heated at 900oC for 90 minutes in a muffle furnace. The formation of crystalline size of SrS phase was evident by the X-ray diffraction analysis of the fired products. The crystallite size of the samples was measured using XRD patterns and found as 69.13nm and 63.68nm respectively. The effects of doping concentrations on luminescent properties of phosphors are investigated, the photoluminescence (PL) excitation spectra of the phosphors are investigated monitoring at 600nm. The emission spectrum of SrS:Eu(0.5%),Ce(0.5%) and SrS:Eu(0.5%),Ce(0.5%),Ga(0.5%) are taken for different excitation wavelengths like 254,262,274,450,470,490,520 and 540nm, It is observed broadband which can be viewed as the typical emission of Eu2+ ascribed to the 4f–5d transitions. Because of their broadband absorption in the region 400–630 nm, The Photoluminescence properties of the materials were studied using Spectrofluorophotometer at room temperature. The Infrared spectra for the prepared solid nanopowders were recorded in the range between 400 and 4000 cm-1 on a Fourier transform spectrometer. The particle morphology of the product was characterized by SEM and particle size analysis.
Keywords |
| Photoluminescence; phosphor rare-earth ions; XRD; solid state reaction technique, LED |
INTRODUCTION |
| Recently various phosphor materials have been actively investigated to improve their luminescent properties and to meet the development of different display and luminescence devices. Inorganic compounds doped with rare earth ions form an important class of phosphors as they possess a few interesting characteristics such as excellent chemical stability, high luminescence efficiency, and flexible emission colors with different activators. There has been much interest in light emitting diodes (LEDs) with emission wavelengths in the ultraviolet-to-infrared range. Major developments in wide band gap III–V nitride compound semiconductors have led to the commercial production of high-efficiency LEDs. Traditional colored LEDs have proven effective in signal applications, as indicator lights, and in automotive lightning. The development of white LEDs as a cost-competitive, energy-efficient alternative to conventional electrical lightning is very important for expanding LED applications toward general white lightning. Phosphors activated with rare earth metal have been widely investigated in the past few decades on account of their technological importance. In particular, phosphors for LED applications have received significant attention in recent years with the rapid development of white LEDs, which have such merits as high efficiency, long lifetime, and low power consumption. Alkali earth sulfide phosphors, such SrS:Eu,Ce and SrS:Eu,Ce,Ga (orange) are also good candidates for LED applications because all of them have strong absorption in the blue region that is suitable to blue. |
| In the present study, we therefore investigated the optical properties of Eu2+ doped alkali earth sulfides, with particular focus on the photoluminescence (PL) characteristics of these phosphors and the color variations of phosphor-converted colored LEDs pumped by blue LEDs. We adopted the standard solid state reaction technique to prepare SrS:Eu,Ce and SrS:Eu,Ce,Ga with good morphologies and fine crystal structures, and its emission and excitation intensity of luminescence were also studied. The present paper reports the Photoluminescence (PL) of the SrS phosphor doped with Eu,Ce and Eu,Ce,Ga rare-earth ions with different emission and excitation wavelengths. |
MATERIALS AND EXPERIMENTAL METHODS |
| All the chemical reagents were analytically pure and used without further purification. SrS:Eu,Ce and SrS: Eu,Ce,Ga(all are 0.5 mol%) phosphor samples were prepared by the conventional solid state reaction method. Strontium Carbonate (SrCO3) and Sulphur(S) were taken as starting compounds in a Stoichiometric proportions of 3:2 to prepare SrS. The starting compounds along with Europium oxide (Eu2O3), Cerium oxide (CeO2) and without and with Gallium oxide (Ga2O3) as a dopant were taken in to an agate mortar and pestle. They were mixed and grounded for one hour to make a fine powder with intermediate mixings and groundings. The obtained powder was taken into a alumina crucible and heated in a muffle furnace under reduced atmosphere at 9000C for one hour. Body color is orange for SrS: Eu, Ce and SrS: Eu,Ce,Ga. All the phosphor samples were characterized by X-ray diffraction using (Synchrotron Beam Indus -II), Particle size analysis was done using laser particle size analysis Malvern Instrument Ltd (U.K). The Photoluminescence (PL) emission and excitation spectra were measured by Spectrofluorophotometer (SHIMADZU, RF-5301 PC) using Xenon lamp as excitation source at display research Lab, Department of Applied Physics, Faculty of Technology and Engg., M.S.University, Baroda. The emission and excitation slit were kept at 1.5 nm, recorded at room temperature. |
RESULTS AND DISCUSSION |
| 1-X-ray Diffraction (XRD) |
| The phase verification, structural parameters and crystalline structure of the SrS: Eu2+, Ce3+ and SrS: Eu2+, Ce3+, Ga3+ phosphors were analyzed by X-ray powder diffraction studies (XRD) of powder phosphors prepared via solid state reaction method and calcined at 900oC under carbon reduction atmosphere, at room temperature XRD graphs were drawn in wide range of Bragg’s angle 2θ from 20 to 50. The crystallite size is calculated using Scherer’s formula d= K.λ/ β cosθ, where ‘K’ is the Scherer’s constant (0.94), ‘λ’ the wavelength of the X-ray (1.54060 Å), ‘β’ the full-width at half maxima (FWHM) (0.1178), ‘θ’ the Bragg angle of the XRD big peak,. Fig-1a is the XRD pattern of SrS: Eu2+, Ce3+ (0.5 mol % of each) phosphor. The calculated crystallite size is found 69.13nm. Fig-1b is the XRD pattern of SrS: Eu2+, Ce3+, Ga3+ (each 0.5 mol %) phosphor. The calculated crystallite size is from the figure more XRD peaks are observed when compared to SrS: Eu2+, Ce3 phosphor (Fig-1a), the main peak is at 25.26o and the second peak at 29.74o followed by many other Bragg’s reflections. The crystallite size is calculated for 25.26o and 29.74o peaks from Scherer’s formula. The average crystallite size from both the peaks is 63.68nm and 51.39nm, from XRD study it appears SrS: Eu2+, Ce3+, Ga3+ might have formed more than one phase. These XRD peaks are well indexed based on the JCPDS No.75-0895. This reveals that the structure of doped (Eu,Ce and Eu,Ce,Ga) phosphor SrS is cubic and is agreement with previous workers. |
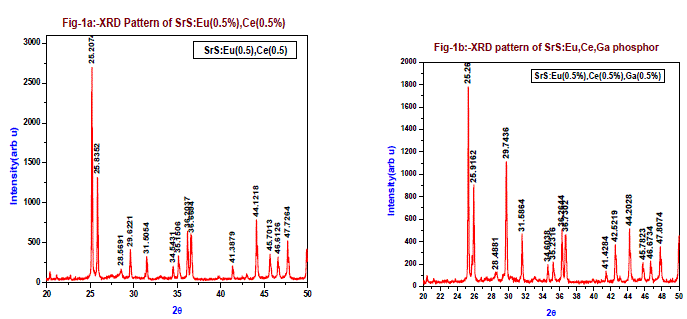 |
2-SEM Analysis |
| Fig-2a and 2b are SEM micrographs of the SrS:Eu2+, Ce3+ (0.5 mol % of each) phosphor. The particles looks agglomerated having size of 1 micron to 10 micron are observed from the SEM graphs. Fig-2c and 2d are SEM micrographs of the SrS: Eu2+, Ce3+,Ga3+ phosphor. The particles look agglomerated having the size of few sub microns to 12 microns are observed from the SEM graphs for different magnifications |
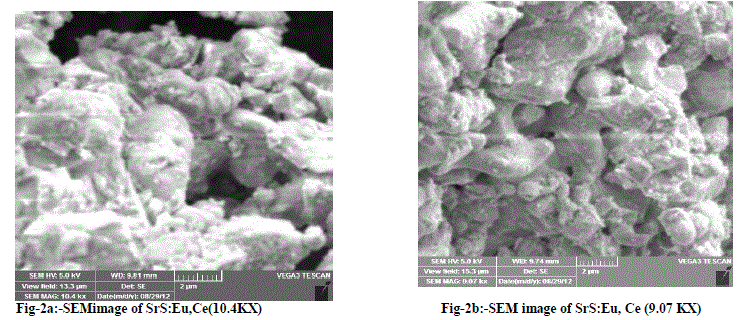 |
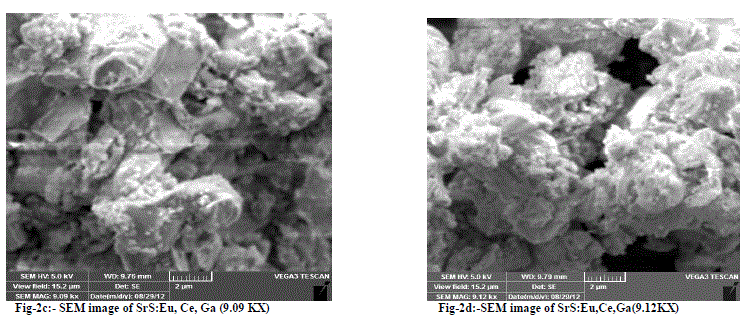 |
| 3-Photoluminescence Study (PL) |
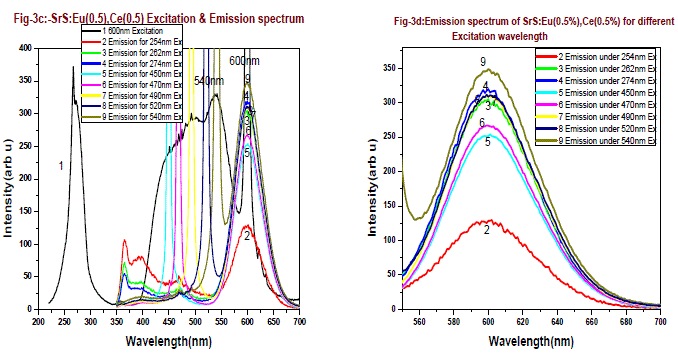
| Fig-3a and 3b are the excitation spectrum of the phosphor SrS:Eu(0.5 mol %), Ce(0.5 mol %) and SrS:Eu(0.5 mol %), Ce(0.5 mol %),Ga(0.5 mol %) monitoring at 600nm. From the figures it is found there are two excitation bands starting from 225 - 325nm, peaking around 254nm and second is from 400 - 570nm. The first band is UV region and second band is perfect blue green visible region, there is also another small absorption band of 365nm, the 365nm band is due to charge transfer transition of Ce3+ from 4f to conduction band. The excitation bands at 254nm and 460nm can be assigned to the eg to 2tg 5d bands of Ce3+. The broad excitation bands of the 600 nm are found at 265 nm and 540 nm, which can be attributed to the (4f7) eg and 4f6 5d1(t2g) field splitting 5d bands of Eu2+ respectively. From these figures the excitation band at 254nm peak intensity decreased by 40% and 540nm peak raised by 38% when Ga is doped in SrS: Eu,Ce phosphor. |
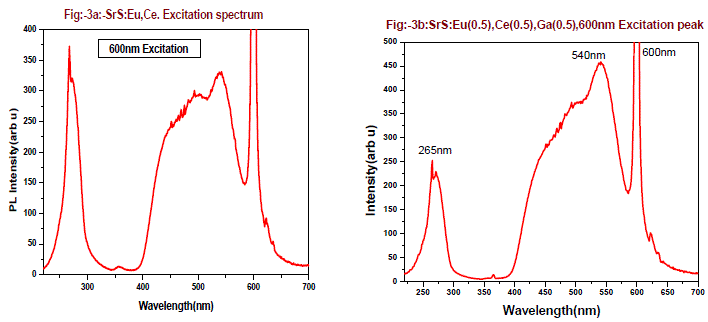 |
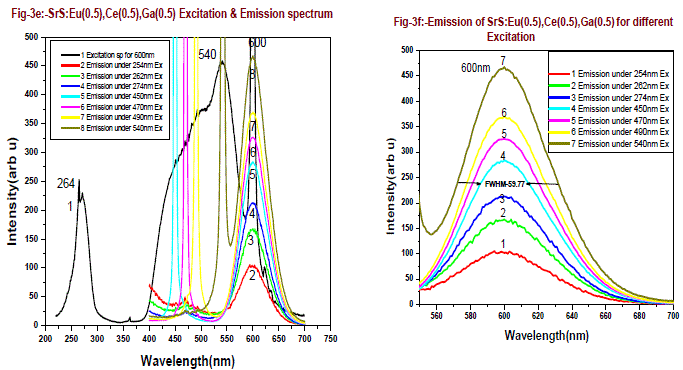
| Fig-3c is the emission and excitation of SrS:Eu(0.5 mol %), Ce(0.5 mol %) phosphor, when excited with 254nm , 262nm, and 274nm, emissions are found at 365nm, 395nm and 467nm with less intensity along with a broad emission peaking at 600nm. The emission peak is found at 600 nm and the excitation bands for this emission observed at 290 nm and 460 nm. The excitation profile is modified from doped SrS:Eu2+, Ce3+ system. As excitation wavelength increases from 254nm to 274nm the emission intensity at 600nm increases from 129 to 320 units, which is 2.5 times more. Since energy of 254nm to 274nm, the energy transfer occurs between Ce3+ to Eu2+, when excitation is from 450nm to 540nm, the emission intensity increased from 255 to 350 units, an increase of 40%. It is found from fig-3d, two absorption bands one is around 460nm to 480nm and another is 530nm to 540nm, this is due to dipole-dipole interaction and higher energy transition rate between Ce3+ to Eu2+. Emission intensity of 600nm peak verses excitation wavelength is presented in fig-3g, The 600nm emission corresponding to the 5D0→ 7F2 transition of Eu2+, is broad emission range from 550-675nm. From the emission spectrum it is observed that the sample shows 600nm emission and the maximum emission intensity for 600nm peak is found for 540nm excitation wavelength. Fig-3e is the emission and excitation of the SrS:Eu(0.5 mol %), Ce(0.5 mol %),Ga(0.5 mol%) phosphor, when the phosphor is excited with 254nm , 262nm, and 274nm, the emissions are found at 467nm with less intensity along with a broad emission peaking at 600nm. The emission peak is found at 600 nm and the excitation bands for this emission are observed at 290 nm and 460 nm. |
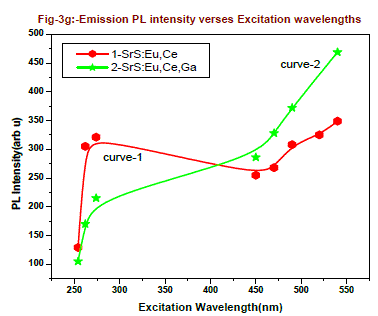
| The excitation profile is modified from doped SrS:Eu2+, Ce3+ ,Ga3+ system. As excitation wavelength increases from 254 - 274nm, the emission intensity of 600nm peak increases from 105 to 215 units, which is around 2 times more since energy of 254nm is more when compared to 274nm, the resonance energy transfer occurs between Ce3+ to Eu2+. When the excitation is from 450nm to 540nm, the emission intensity increased from 286 to 469 units, an increase of 80%. Two absorption bands are observed, one is around 460nm to 490nm and another is 530nm to 545nm, this is due to dipole-dipole interaction and resonance energy transition rate between Ce3+ to Eu2+. Emission intensity of 600nm peak verses various excitation wavelengths are presented in fig-3g and table-1 for better comparison. From figure-3g and table it is found that the emission intensity of 600nm peak gradually increases as increase in excitation wavelength, The 600nm emission corresponding to the 5D0→ 7F2 transition of Eu2+, which is a broad emission range from 550-675nm. From the emission spectrum it was observed the maximum emission intensity for 600nm peak is found for 540nm excitation wavelength. Full width at half-maximum (FWHM) is around 60nm.From fig-3g we conclude that the resonance energy transfer from Ce+3 to Eu+2 is more when Ga is doped in the SrS:Eu+2,Ce+3 in the higher Excitation wavelengths 450, 470,490,520 and 540nm it shows curve-2. From overall discussion SrS:Eu2+, Ce3+,Ga3+ phosphor one can normally conclude that presence of Ga emission of PL intensity by 30% increase. The preference of more than one phase but did not influence the PL emission peak shape of 600nm. |
 |
 |
| 4-Particle size analysis |
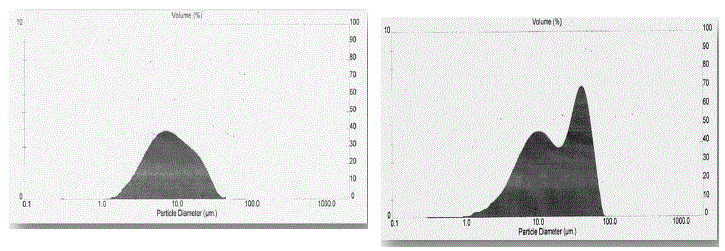
| The Particle size distribution histograms of the SrS:Eu2+, Ce3+ (0.5 mol %) phosphor particles as shown in the Fig- 4a.The prepared phosphor specimen particle size was measured by using laser based system Malvern Instrument U.K. From the figure it is found the average particle size is 4.5 μm, the average surface area 1.341 M2/gm. |
 |
| The Particle size distribution histograms of the SrS:Eu2+, Ce3+,Ga3+ (each 0.5 mol %) phosphor particles as shown in the Fig-4b. The prepared phosphor specimen particle size was measured by using laser based system Malvern Instrument U.K. From the figure it is found there are two peaks one is at 10 μm and another is at 50 μm are observed, and the average surface area is found to be 1.3974 M2/gm. From XRD study we presume two phases of SrS:Eu2+, Ce3+,Ga3+ phosphor and particle size distribution study two major distributions are observed which are around 10 μm and 50 μm, this also suggest the presence of two phases. SEM study suggests presence of sub microns to few microns particles of the phosphor is also suggested more than one phase. From overall discussion SrS:Eu2+, Ce3+,Ga3+ phosphor one can normally concluded that presence of Ga emission of PL intensity by 30% increase. The preference of more than one phase but did not influence the PL emission peak shape of 600nm. |
| 5-FTIR study |
| In order to determine the chemical bonds in a molecule FTIR analysis was carried out. Fig-5a is the FTIR of the SrS:Eu2+, Ce3+ (0.5 mol %) phosphor, the main absorption around 3400 are assumed H-O-H stretching followed by other bonds of Sr-S stretching, Sr-O stretching and CO-OH stretching. CO-OH and H-O-H stretching are due to absorbed CO2 and H2O molecules from atmosphere. |
 |
| Fig-5b is the FTIR of the SrS:Eu2+, Ce3+, Ga3+ ( each 0.5 mol %) phosphor, the main absorption around 3400 are assigned to H-O-H stretching followed by other bonds of Sr-S stretching, Sr-O stretching, Sr-S stretching, Eu-O stretching and CO-OH stretching. CO-OH and H-O-H stretching are due to absorbed CO2 and H2O molecules from atmosphere. |
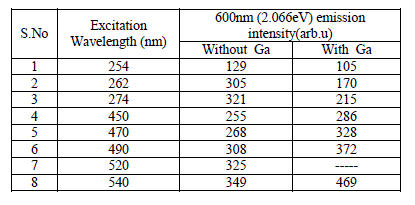 |
| Table-1: Emission intensity of 600nm peak for different Excitation wavelengths without and with Ga co-doped SrS:Eu Ce phosphor. |
CONCLUSION |
| It is concluded that the presence of gallium in SrS:Eu,Ce did not play any role to give green emission of gallium. SrS: Eu,Ce and SrS: Eu,Ce,Ga phosphors are synthesized in reducing atmosphere have been stabilized in 2+ states of Europium which emitted a broad emission from 550nm to 680nm with a peaking around at 600nm. From the excitation studies of the Phosphor, the phosphor can be excited from 240nm to 280nm with high intensity along with a peak at 470nm followed by a broad emission with a peaking 600nm. For the SrS: Eu,Ce,Ga phosphors, the average crystallite size from both the peaks are 63.68nm and 51.39nm, This phosphor SrS:Eu,Ce,Ga can be considered as good material for CFL, FL as green-red emitting material, these phosphors meet the application requirements for blue LED chips because all of them have strong absorption in the blue region that is suitable to blue. |
ACKNOWLEDGEMENT |
| The author Sayana.K R is gratefully thanking the Secretary and Correspondent of QIS College of Engineering and Technology, Ongole, A.P, India, for continuous supporting and encouragement to my research work. |
References |
|