ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Indravathi.G1, Kiran Kumari.K2, Bhuvaneswari Devi.C3 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Manganese (Mn) is a mineral element is nutritionally essential and potentially toxic to biological tissues. Due to pollution Mn level is potential in environment. In the present study Albino rats were exposed to manganese and α- tocopherol to observe the possible interactions. Albino rats of two months and four months were exposed to Mn by intraperitoneal injections dissolved form of Manganese sulphate (MnSo4). α- tocopherol was given to the Mn exposed rats through intraperitoneal injections (30mg/Kg bw). Rats were randomly divided into five groups with different treatments such as Controls, Low Mn exposure (2.5 mg/kg bw), Low dose of Mn exposure along with α- tocopherol, High dose of Mn exposure(5mg/kg bw), High dose of Mn exposure along with α- tocopherol in two different age groups two months and four months. Hematological changes were observed after Mn exposure. In this study, we have examined that the red blood cells, white blood cells, hemoglobin, packed cell volume, serum urea, serum creatinine, serum cholesterol, serum bilirubin. Hematological changes in low and high dose of Mn exposure showed decrease in Red blood cells, hemoglobin, packed cell volume and increase in white blood cells, serum urea, serum bilirubin, serum creatinine, and serum cholesterol in dose dependent manner. α- tocopherol is considered the most effective liposolouble antioxidant. However, the animals which received α- tocopherol with low and high dose of Mn showed recovery. The two months rats showed more susceptibility to Mn and also showed more recovery with α- tocopherol when compared to four months rats.
Keywords |
| Manganese, α- tocopherol, blood, serum. |
1. INTRODUCTION |
| Manganese (Mn) is an essential trace element in humans that can elicit a variety of serious toxic responses upon prolonged exposure to elevated concentration either orally or by inhalation [1]. The importance of hematological parameters in clinical biochemistry is well established. Recent speculations have proved that they may be used as valuable indicators of disease or stress in animals [2]. Blood parameters are probably the more rapid and detectable variations under stress and are useful in assessing the health condition [3]. Hematological values are widely used to determine systematic relationship and physiological adaptations including the assessment of general health conditions [4]. Hence, hematological investigations have long been used as diagnostic tools to investigate pathological, physiological and metabolic alterations ([5], [6]). Manganese (Mn) plays an essential role in regulation of cellular energy, bone and connective tissue growth and blood clotting [7]. The concentration of manganese present in the CNS may be affected in the presence or absence of iron. Iron homeostasis is thought to play a crucial role in manganese uptake, regulation and transport [8]. Iron can be implicated as a catalyst for lipid peroxidation either as heme proteins or in non heme forms ([9], [10]). An iron-responsive anemia is commonly found with orally-induced manganese toxicity [11]. High concentrations of iron lead to a lower absorption of manganese, while low levels of iron promote manganese absorption ([12],[13],[14],[15],[16],[17]). Manganese has been reported to have a toxic effect on cardiac cells and tissues from animals in vitro but this has not been demonstrated in whole animals or humans [18]. Some studies have reported that blood Mn was associated with exposure and adverse neurological effects. ([19],[20],[21],[22],[23],[24],[25]). .Mn has a half-life in blood of 10 to 40 days [26]. Blood Mn may also be influenced and change as a result of dietary intake, which may also confound study results [27]. Another study performed in blood and breast milk samples amongst plant and non- workers concluded that levels of manganese were found to be greatest in both biological matrices in plant workers, more than mercury and lead [28].Welders had significantly higher levels of Mn in blood and hair than the control group7.6 and 3.2 times higher, respectively. A recent study by Montes et al [29] evaluated the relationship between blood Mn and prolactin as marker of effect in subjects living in a mining district in central Mexico environmentally exposed to Mn. Deschamps et al concluded that the biological significance of Mn in blood is far from clear[30]. Normal whole blood levels of Mn (MnB) range from 7-12 μ g/l and 0.6 to 4.3 μ g/l in serum [31]. |
| Clinical Mn neurotoxicity has been reported in patients receiving long-term parenteral nutrition and in patients with chronic liver dysfunction or renal failure, as a result of their inability to eliminate and clear Mn from the blood ([32], [33], [34],[35], [36]). Vitamin E is known to have been proven beneficial in some disease processes. It protects the body’s biological systems [37] by preventing lipid peroxidation [38]. In the context of the above studies we have focused our studies to examine the alterations in the blood in low and high dose of Mn exposed rats and possible recovery of toxicity with α- tocopherol in two months and four months old rats. |
II. MATERIALS AND METHODS |
| A. Procurement and maintenance of experimental animals: Young albino rats (Wister) were purchased from Sri Venkateswara trades, Bangalore and maintained in the animal house of Watson Life Sciences, Tirupati. The animals were housed in clear plastic cages with hardwood bedding in a room maintained at 28º ± 2º C and relative humidity 60 ± 10% with a 12 hour light/day cycle. The animals were fed in the laboratory with standard pellet diet supplied by Sri Venkateswara traders, Bangalore and water ad libitum. |
| Albino Wister rats were exposed to low dose (2.5 mg/kg bw) and high dose (5 mg/kg bw) of Mn intra peritonially daily for a period of 4 weeks. The supplementation lasted for four weeks and after the last day; the animals were fasted overnight and an aesthesized by dropping each in a transparent plastic jar saturated with chloroform vapor. Blood sample was collected through cardiac puncture and divided into plain and EDTA-containing centrifuge tubes. The hematological changes in control, Mn exposed and α-tocopherol supplemented 2 months and 4 months old rats were observed in the blood and serum. |
| B. Chemicals: Mn and α- Tocopherol was selected as a test chemicals. The chemicals used in study namely R.B.C fluid, W.B.C fluid, hydrochloric acid, sodium citrate, Isopropanal. |
| Estimation of red blood corpuscles:RBC count was made with a Neubauer crystalline chamber as described by Davidson and Henry (1969) [39]. The blood was drawn up to 0.5 marks in RBC pipette and immediately the diluting fluid was drawn up to 100 ml mark (thus the dilution is 1:200). The solution was mixed well by shaking gently. It was allowed to stand for 2 to 3 minutes. The counting chamber and cover glass were cleansed and cover slip was placed over the ruled area. Again the solution was mixed well by shaking gently and stem full of solution was expelled and a drop of fluid was allowed to flow under the cover slip holding the pipette at an angle of 400. It was allowed to stand for 2 to 3 minutes to allow RBC to settle. Afterwards the ruled area of the counting chamber was focused under the microscope and the number of RBC’s were counted in five small squares of the RBC column under high power and the number of RBC per Cu.mm were calculated accordingly. |
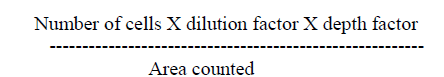 |
| Estimation of Hemoglobin concentration (Hb): The Hemoglobin concentration (Hb) was estimated by acid haematin method [40]. N/10 hydrochloric acid was taken up to 10.0 marks in a graduated tube. Blood was collected directly in to the Hb pipette and was transferred into the graduated tube containing N/10 hydrochloric acid. It was allowed to stand for 10 minutes after thorough mixing. Then N/10 hydrochloric acid was added drop by drop, mixing between each addition until the blood colour matched with standard colour. Then the amount of Hb was read from the scale on the graduated tube and the Hb concentration was expressed in grams per cent. |
| Estimation of packed cell volume (PCV): PCV was estimated by micro haematocrit method [41]. The blood was drawn into capillary tube containing the anticoagulant, by capillary action to ¾ of the length. The tubes were tapped to permit blood flow towards an end and to provide sufficient space to prevent outflow and the opposite ends were sealed. The outside of the capillary tubes were wiped free of blood and the index finger was placed over the moist ends to hold the column of the blood in place as the opposite dry ends were forced into the sealing material to form a tight plug. The capillary tubes were placed in the centrifuge with the sealed ends pointing outward and centrifuged at 12,000g for 2 minutes. PCV was determined by rolling the capillary tubes on a reader card until the top of the plasma column was aligned with 100% line and the bottom of the packed erythrocytes was on the zero line. The line that crossed the top of the packed erythrocyte column represented the PCV in percent. |
| White blood corpuscle (WBC) count:Blood is drawn from the vial into WBC pipette up to 0.5 marks and immediately the diluting fluid is drawn up to the mark 10. The solution is mixed thoroughly by shaking. The rest of the procedure is the same as described for RBC. In case of WBC, count was made in bigger squares of the chamber. The WBC count was expressed in number of WBC/ Cu.mm. |
| Estimation of serum total cholesterol: |
| Serum cholesterol was estimated by the method of Natelson (1971)[42]. To 1 ml of serum, 1 ml of isopropanol was added and the contents were centrifuged at 1000g for 15 minutes. 0.5ml of supernatant was taken and to it, 4ml of cholesterol reagent was added. Then the contents were heated at 90ïÃâðC for 15 minutes. After cooling, the samples were read at 560 nm in a spectrophotometer against the reagent blank. Values are expressed in mg/100ml |
| Estimation of Urea: |
| To estimate serum urea we used Bio Systems Analyzer, Spectrometer or Photometer able to read at 340nm [43] |
| Estimation of Creatinine: |
| To estimate serum Creatinine we used Bio Systems Analyzer, Spectrometer or Photometer able to read at 500±20nm. Thermostatic water bath at 370c [43]. |
| Estimation of bilurubin: |
| To estimate serum bilirubin we used Bio Systems Analyzer, Spectrometer or Photometer able to read at 500±20nm. Thermostatic water bath at 370c [43]. |
III. RESULTS |
| A. Estimation of red corpuscle (RBC): In the present study, the levels of RBC increased progressively in two months and four months control rats (Fig 1.1). From the results obtained it is clear that Mn-exposure induced a decrease of RBC levels in rats. The decrease was more prominent in high dose Mn-exposed rats as compared to low dose Mn exposure. It is also clear that the alterations in RBC levels were progressively decreased in two months Mn-exposed rats (in both low and high dose exposure), and showed a marginal decrease in four months rats. |
| The administration of α- tocopherol produced recovery from the Mn toxicity. Protection offered by α-tocopherol was greater in low dose Mn-exposure over high dose Mn-exposure (Fig 1.1). At the same time two months rats showed more recovery in RBC levels with α-tocopherol administered rats was higher as compared to four months rats (Fig 1.1). |
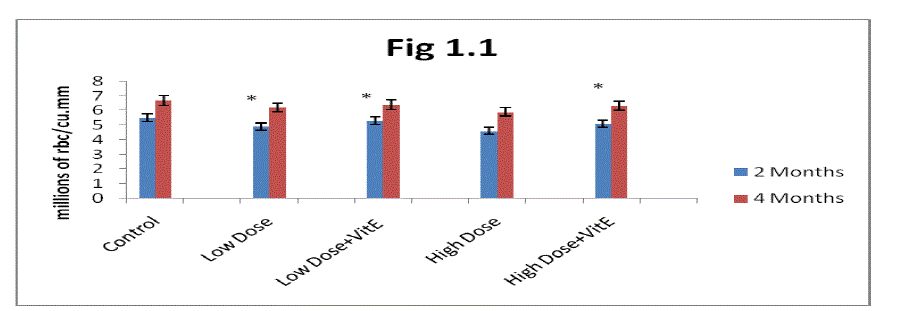
| B. Estimation of Hemoglobin (Hb): From the results presented in (Fig 1.2) it is clear that the Hb concentration progressively increased in two months and four months control rats. Mn-exposure decreased Hb levels in two months and four months rats. The decrease was greater in high dose Mn-exposed rats as compared to low dose Mn-exposure. Alterations in Hb levels were progressively decreased in two months exposed rats in both low and high dose exposure and showed a marginal decrease in four months rats. |
| The administration of α- tocopherol produced recovery from the Mn toxicity. Protection offered by α-tocopherol was greater in low dose Mn exposure over high dose of Mn exposure (fig 1.2). At the same time two months rats showed more recovery in Hb levels with α- tocopherol administrated rats was higher as compared to four months rats. |
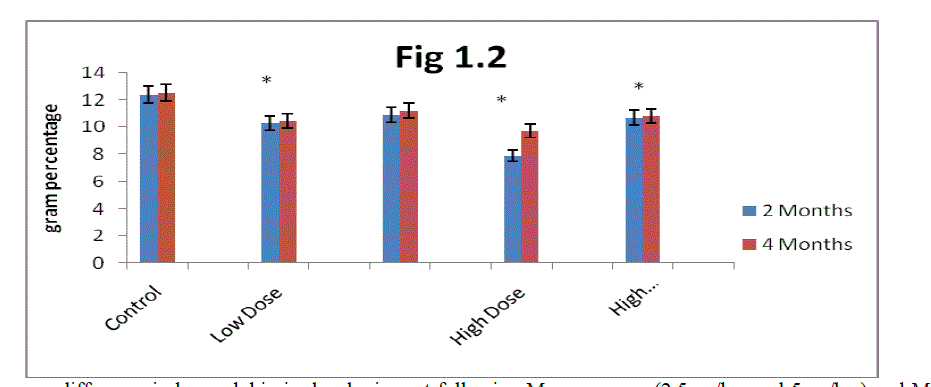
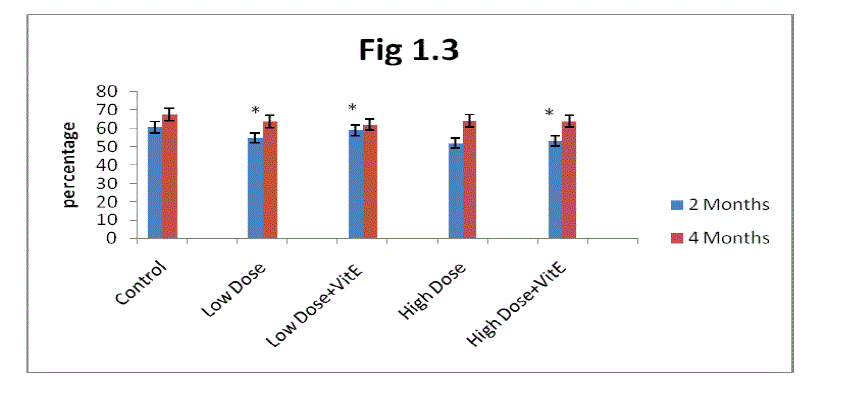
| C. Estimation of Packed cell volume (PCV): From the result present In (Fig 1.3) it is clear that the packed cell volume gradually increased in two months and four months control rats. Mn exposure decreases packed cell volume in two months and four months rats. The decrease was greater in high dose Mn exposed rats as compared to low dose Mn exposure. Alterations in packed cell volume progressively decreased in two months rats in both low and high dose exposure and showed marginal decrease in four months rats. |
| The administration of α-tocopherol produced recovery from the Mn toxicity. The effect of α-tocopherol was greater in low dose Mn-exposed rats as compared to high dose Mn exposure (Fig 1.3). The recovery in PCV in the α-tocopherol administered developing rats was higher in two months rats as compared to four months rats. |
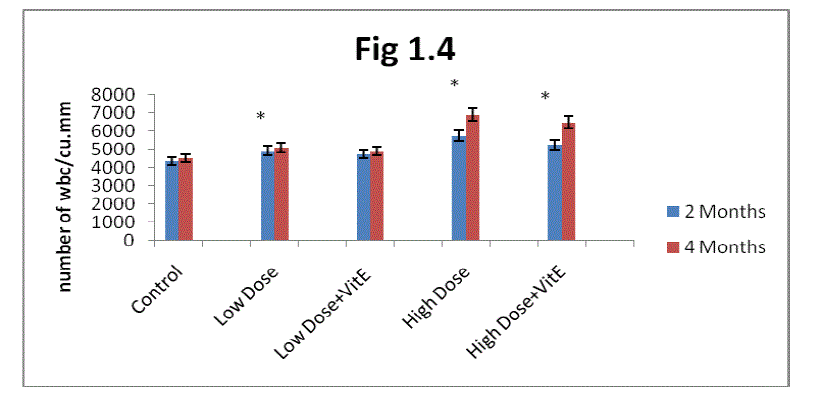
| D. Estimation of white blood corpuscle: From the result present in the (fig1.4) it is clear white blood cell were increased their number in two months and four months rats more than control when exposed to Mn. The increase was greater in high dose-exposed rats as compared with low dose exposure. Maximum increase in WBC was observed in two months rats as compared to four months rats. |
| The administration of α-tocopherol produced reversal from the Mn induced toxic effect. Protection offered by α- tocopherol was greater in low dose Mn exposure over high dose Mn exposure (Fig 1.4).The recovery in white blood cells in the α- tocopherol administrated developing rats was higher in two months rats as compared to four months rats. |
| E. Estimation of total cholesterol: From the result present in the (fig1.5) it is clear cholesterol increased in two months and four months more than control when exposed to Mn. The increase was greater in high dose exposure when compared to low dose exposure. Maximum cholesterol increase observed in two months rats as compared four months rats. |
| The administration of α-tocopherol produced recovery from Mn induced toxicity. Protection offered by α- tocopherol was greater in low dose Mn exposure over high dose Mn exposure (fig1.5). The recovery in cholesterol with the α- tocopherol administrated developing rats was higher in two months rats as compared to four months rats. |
| Estimation of urea: From the result present in the (fig 1.6) it is clear urea levels were increased in two months and four months rats more than control when exposed to Mn. The increase was greater in high dose exposure when compare to low dose exposure. Maximum urea increase observed in two months rats as compared to four months rats. |
| The administration of α-tocopherol produced reversal from the Mn induced toxic effect. Protection offered by α- tocopherol was greater in low dose Mn exposure over high dose Mn exposure (Fig 1.6).The recovery in urea with the α- tocopherol administrated developing rats was higher in two months rats as compared to four months rats. |
| Estimation of Creatinine: From the result present in the (fig1.7) it is clear creatinine gradually increases in two months and four months rats more than control when exposed to Mn. The increase was greater in high dose exposed rats as compared with low dose exposure. Maximum increase in Creatinine was observed in two months rats as compared to four months rats. |
| The administration of α-tocopherol produced recovery from the Mn toxicity. The effect of α-tocopherol was greater in low dose Mn-exposed rats as compared to high dose Mn exposure (Fig 1.7). The recovery in Creatinine with the α-tocopherol administered developing rats was higher in two months rats as compared to four months rats. |
| F. Estimation of Bilirubin: : From the result present in the (fig1.8) it is very clear Bilurubin levels were increased in two months and four months rats more than control when exposed to Mn. The increase was greater in high dose-exposed rats as compared with low dose exposure. Maximum increase in bilirubin was observed in two months rats as compared to four months rats. |
 |
 |
 |
 |
| Fig (1.8) mean difference in bilirubin in developing rat following Mn- exposure (2.5mg/bw and 5mg/bw ) and α- tocopherol administration. Each bar represents mean ± SD (n=6). The values marked with asterisk (*) are significantly different from control at p |
| The administration of α-tocopherol produced reversal effect from the Mn induced toxic effect. Protection offered by α- tocopherol was greater in low dose Mn exposure over high dose Mn exposure. The recovery in bilirubin with the α- tocopherol administrated developing rats was higher in two months rats as compared to four months rats. |
IV. DISCUSSION |
| Manganese is a ubiquitous element that is essential for normal physiologic functioning in all animal species. Several disease states in humans have been associated with both deficiencies and excess intakes of manganese. Blood samples were assayed for several hematological parameters (erythrocyte count, leukocyte count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, platelets, and differential leukocyte count). |
| Several authors reported decreased RBC count, Hb levels and PCV in different animals exposed to pesticides, heavy metals and toxins ([44],[45], [46] [47]). It is predicted that High concentration of cerium oxide nanoparticles, reduced the number of blood cells due to inhibition of cell activity, antimiotic properties and also stimulation of oxidative stress in cells, reduction of cellular antioxidants and Increasing of involvement cells in the immune processes. In addition to the central nervous system effects, an iron -responsive anemia is commonly found with orally-induced manganese toxicity [48] |
| Lambs on a high manganese diet developed a reduction in hemoglobin. This observation is consistent with the anemia seen in humans and indicates that large amounts of manganese can interfere with intestinal iron absorption [49]. Large levels of manganese intake by calves can reduce growth rate and hemoglobin level and it is also known to interfere with the utilization of cobalt and zinc in ruminants [50]. |
| Our study showed decrease in hemoglobin level with intra peritoneal administration of Mn, these results may be attributed to the following reasons. Hematological indices vary from animal to animal and in some animals at different stages of life. Age related hematological changes in cotton rats (Sigmodan hispidus) were reported by Maity and Guru (1998) [51]. Red blood corpuscle (RBC), Hemoglobin (Hb), Hematocrit (HCT) in the blood of mammals are reported to vary with sex [52], age [53], body weight([54],[55]), size [56], nutritional status [57], season [58], habitat, and altitude [59],In our studies we measured age (2months and 4months) related hematological changes. These changes showing similarity with above sentence. |
| The increase in WBC reported in rats treated with environmental toxicants such as in phorate (insecticide) [60], Phenylhydrazine [61], fenvelarate [62], and aluminium [63]. Similar increase in WBC was observed in fish exposed to various pollutants including metals [64], [65]). Our study supports the similar increase in white blood cells with intra peritoneal administration of Mn. |
| Cholesterol levels in plasma and liver decreased in a dose and time dependent manner when vitamin E was administered to deficient animals [66]. Increased cholesterol levels were reported in various stress conditions [67] during exercise training [68], and with guanidine hydrochloride (GuHcl) treatment ([69], [70]). Serum cholesterol concentrations greatly decreased with increase in vitamin E in the diet [71]. |
| Normal adult body pool of about 20 mg is maintained by the liver, and excess manganese is excreted into the intestine via the bile. This control is achieved with a daily intake of 10 to 20% of the total pool; therefore, relatively large amounts are handled by this mechanism. The normal urinary level of manganese averages about 2.75 μg/L with a range of about 1.0 to 8.0 μg/L. Urinary levels over 10 μg/L are indicative of manganese overexposure [49]. In our studies also we observed Mn exposed rats two months and four months rats showing more bile in excretion respectively than control rats of two months and four months. Vitamin E is known as one of the most potent natural lipophilic antioxidants, In many studies vitamin E neutralizes lipid peroxidation and unsaturated membrane lipids because of its oxygen scavenging effect ([72] ,[73]). |
V. CONCLUSION |
| Manganese is an essential metal potentially toxic to animal tissues. In this study it was clear control animals showed higher red blood cells, hemoglobin, packed cell volume, while the manganese exposed rats to low and high dose showed decrease in above parameters. At the same time white blood cells, urea, bilirubin, cholesterol were increased than control. It can be concluded from present results that low dose and high dose of Mn alter hematological changes in dose depending manner. The α- tocopherol showed reversal effect to Mn toxicity in blood cells and serum composition as effective chelating agent, hence there is a decreased effect of manganese and increased blood and serum composition levels in the manganese exposed rats treated with α-tocopherol. |
| Acknowledgements: This work was supported by DST grant No. SR/FT/LS-103/2009 (G), Govt. of India |
References |
|