Diagnosis of melanocytic tumors poses two big problems for a histopathologist, firstly to diagnose it as melanocytic tumor and the other important problem is the determination of whether the lesion of obvious melanocytic nature is benign or malignant. Here we wish to provide few key histopathological features of some important tumors that will guide a histopathologist in overcoming these problems. Melanocytes are generally positive for melanin stains (such as the Fontana- Masson silver stain), tyrosinase, DOPA reaction, S-100 protein, NSE, Mart-1/MelanA (A103), microphthalmia transcription factor, Sox10 (a neural crest transcription factor) PAX3 (a transcription factor with an important role in the development of melanocytes during embryogenesis), and vimentin; the intensity of these reactions shows marked variability, possibly depending on the functional status of the cell.1 Stains for neurofilaments and glial fibrillary acidic protein are negative. Stains for HMB-45 are generally negative in normal resting melanocytes but positive in activated melanocytes, including those of malignant melanoma. Keratin is another marker that is consistently absent in normal melanocytes but sometimes expressed in their neoplastic counterpart. The fact that normal melanocytes show strong immunoreactivity for the BCL2 proto-oncogene product has been recorded. This is also expressed in benign nevi and less commonly in malignant melanomas. Though it is a challenge for a histopathologist to make a specific diagnosis of a case of melanocytic tumor but with clinical correlation and step by step approach aided with IHC, most of the tumors can be diagnosed correctly. Staging of the tumors is obviously done after proper consultation with dermatologist
Keywords |
| Melanoma, nevus, HMB-45, Melan-A. |
Introduction |
| Diagnosis of melanocytic tumors poses two big problems for a histopathologist, firstly to diagnose it as melanocytic tumor and the other important problem is the determination of whether the lesion of obvious melanocytic nature is benign or malignant. Here we wish to provide few key histopathological features of some important tumors that will guide a histopathologist in overcoming these problems. |
| Melanocytes are the cells derived from neural crest and are located in the basal layer of skin, hair follicles, most squamous-covered mucosal membranes, leptomeninges, and several other sites. Theirfunction is to produce an insoluble pigment known as melanin. |
| Melanocytes are generally positive for melanin stains (such as the Fontana–Masson silver stain), tyrosinase, DOPA reaction, S-100 protein, NSE, Mart-1/MelanA (A103), microphthalmia transcription factor, Sox10 (a neural crest transcription factor) PAX3 (a transcription factor with an important role in the development of melanocytes during embryogenesis), and vimentin; the intensity of these reactions shows marked variability, possibly depending on the functional status of the cell.[1] Stains for neurofilaments and glial fibrillary acidic protein are negative. Stains for HMB-45 are generally negative in normal resting melanocytes but positive in activated melanocytes, including those of malignant melanoma. Keratin is another marker that is consistently absent in normal melanocytes but sometimes expressed in their neoplastic counterpart. The fact that normal melanocytes show strong immunoreactivity for the BCL2 proto-oncogene product has been recorded. This is also expressed in benign nevi and less commonly in malignant melanomas [2]. |
Classification |
| Tumors of the melanocytes can be broadly divided as: |
| Melanocytic nevi |
| • Congenital melanocytic nevus |
| • Acquired melanocytic nevus |
| • Junctional, Intradermal and Compound nevus |
| • Balloon cell nevus |
| • Halo nevus |
| • Cockade nevus |
| • Deep penetrating Nevus |
| • Spitz nevus and related nevus |
| • Blue nevus and related nevus |
| • Dysplastic nevi / Clinically atypical nevus |
| Malignant melanoma of the skin (melanoma) including specific histogenetic types |
Histopathology with key clinical features |
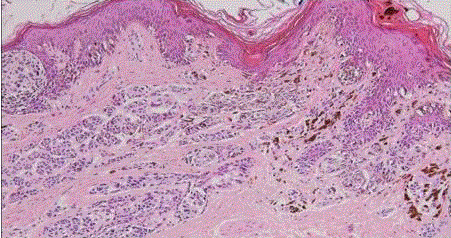
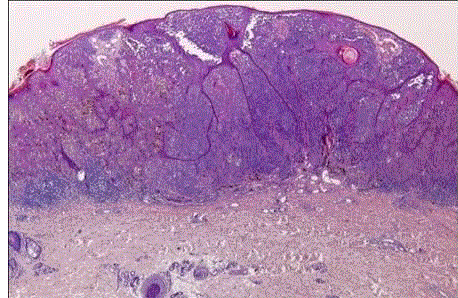
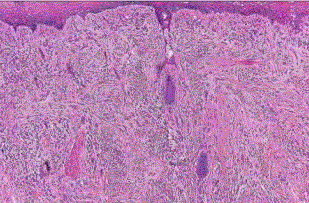
| Congenital nevus: they present at birth and differ from the more common acquired variety because of its generally larger sizeand it has a tendency to involve the reticular dermis and subcutaneous tissue. There is single cell permeation of dermal collagen bundles. Involvement of skin adnexa, arrector pili muscles, nerves, and vessels are more commonly seen in congenital nevi than other melanocytic tumors [4]. In congenital nevus, the neval cells are found deeper into the dermis. Also, the deeper nevus cells can be found along with neurovascular bundles. (fig 1) |
| Congenital melanocytic nevi may be further divided into the following types:[5] |
| • Small-sized congenital nevus - having a diameter less than 2 cm. |
| • Medium-sized congenital nevus - having a diameter more than 2 cm but less than 20 cm. |
| • Giant congenital nevus (Bathing trunk nevus / Garment nevus / Giant hairy nevus / Nevus pigmentosus et pilosus) – have one or more large, darkly pigmented and sometimes hairy patches.[6] |
| Junctional, Intradermal and Compound nevus: they are classified according to the location of the nevus cells in relation to the major epidermal and dermal landmarks. |
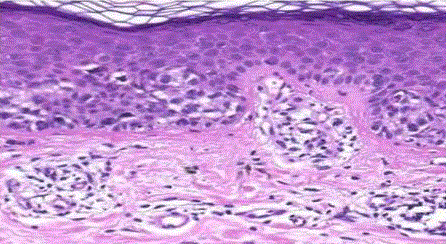
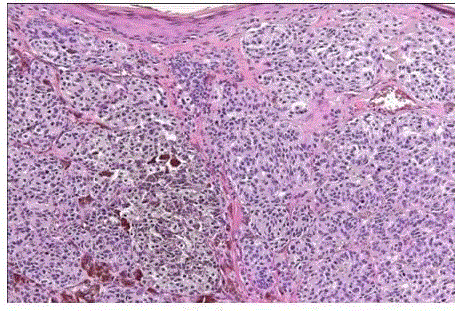
| Junctional nevus: in which the melanocytic proliferation is restricted to the basal portion of the epidermis (‘junctional’ area). Nevi of the palms and soles are nearly always of the junctional type. Microscopically, it is characterized by the presence of melanocytic nests (theques) on the epidermal side of the dermoepidermal junction (fig 2). |
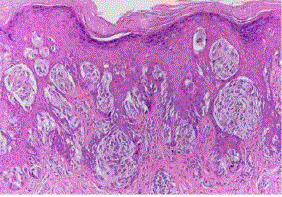
| Intradermal nevus: in which all the melanocytes are in the dermis. This is the common adult type of nevus. It may be papillomatous, pedunculated, or flat, and it is often hairy. Microscopically, small nests or bundles of melanocytes are seen in the upper dermis, with a tendency to concentrate around pilosebaceous units [7]. (fig 3). |
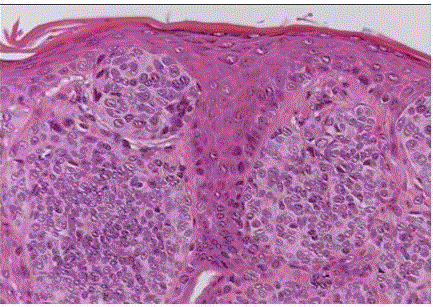
| Compound nevus: it combines the features of both junctional and intradermal types, i.e., it has both an epidermal and a dermal component. (fig 4) |
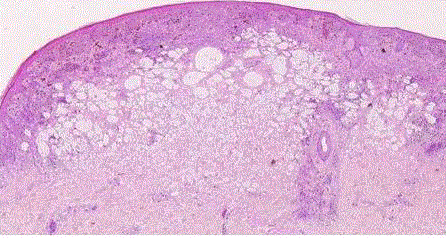
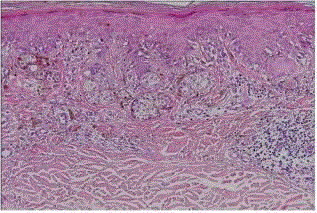
| Balloon cell nevus: it is a rare variety of nevus that is identified by the presence of large pale melanocytes with foamy cytoplasm (fig 5). This is perhaps the result of a biochemical alteration in melanin synthesis. Balloon cells also can occur in blue nevi and malignant melanomas [8]. |
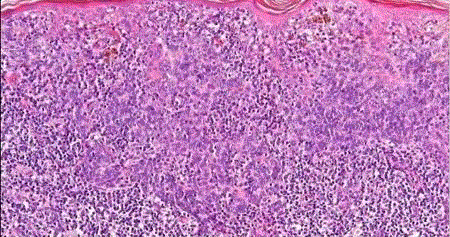
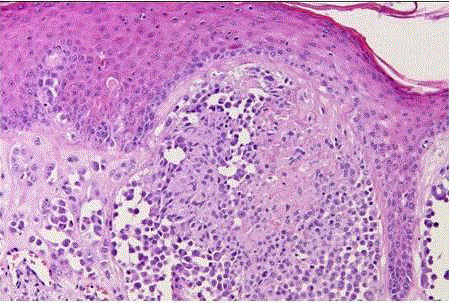
| Halo nevus (leukoderma acquisitum centrifugum): it represents a melanocytic nevus that is surrounded by a zone of depigmented skin. It is most commonly found on the trunk of young patients, and it can be multiple [9]. Microscopically, it is characterized by a heavy infiltration of the nevus by lymphocytes and histiocytes (fig 6) and is thought to represent the expression of a host immune response. |
| Cockarde nevus: it is characterized clinically by a concentric pattern of pigmentation characterized by a central pink to darkly pigmented papule that is surrounded by a stippled pigmented circle, with a clear zone in between [10]. Microscopically, the central lesion is a junctional or compound nevus without inflammatory changes and the peripheral portion (ring) shows multiple junctional melanocytic nests. The intermediate zone is basically unremarkable. |
| Deep penetrating nevus: it is a variant of compound nevus in which the dermal component extends deeply into the reticular dermis and sometimes even into the subcutaneous fat. The junctional component is usually inconspicuous, whereas the dermal component is cellular, nested or fascicular with abundant pigmentation, and cytologically showing mild atypia. Mitoses are nil, and there is little or no inflammatory infiltrate (fig 7). Sometimes deep penetrating nevus is seen admixed with other types of nevi, as a form of combined nevus [11]. |
Spitz nevus and related nevus |
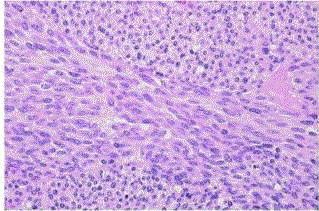
| Spitz nevus (spindle and/or epithelioid cell nevus): Characteristically occurs before puberty, but it may also appear in adult life [12]. They typically show a raised, pink or red nodule in the skin of the face not too different from a hemangioma. Microscopically, most Spitz nevi are of the compound type, with a prominent intraepidermal component. About 5–10 % are junctional, and 20% or more are intradermal [13]. They are composed of spindle cells, epithelioid cells, or an admixture of both. |
| The spindle cell variant is characterized by cigar-shaped cells with large nuclei and prominent nucleoli (fig 8). The cells of the epithelioid type have similar nuclei and a large, polygonal cytoplasm with distinct borders (fig 9). A variant of the epithelioid type is a multinucleated giant melanocyte containing up to 10–20 nuclei. Mitoses are found in approximately half of the cases [14]. but atypical mitoses are exceptional. Pigmentation is usually scanty, but a deeply pigmented variant (located on the proximal extremities of young adults) exists, that is referred to as pigmented spindle cell nevus or Reed nevus (fig 10) and it is also regarded by most authors as a separate entity from Spitz nevus [15]. |
| Desmoplastic Spitz nevus: It is characterized by the presence of extensive stromal desmoplasia in Spitz nevi of predominantly dermal location, which encircles the individual cells and simulates an invasive pattern.[16] This feature is more commonly seen in adults [17]. |
| Some other features that can occasionally be seen in a case of Spitz nevi are predominantintraepidermal growth (pagetoid Spitz nevus),[18] invasion of lymph vessels, [19] florid pseudoepitheliomatous hyperplasia,[20] ‘tubular’ pattern of growth,[21] plexiform pattern of growth (plexiform Spitz nevus),[22] halo reaction, [23] and prominent vasculature (angiomatoid Spitz nevus) [24]. |
| Spitz nevi can sometimes be confused with malignant melanoma histologically. Features that favor a diagnosis of Spitz nevus over malignant melanoma are symmetric shape, sharp lateral demarcation, maturation in depth, arrangement of the spindle cells perpendicularly to the skin surface, presence of tadpole and multinucleated giant cells, lack of upward epidermal spread, presence of telangiectasia, edema, fibrosis, presence of eosinophilic hyaline bodies along the dermoepidermal junction thought to be made of basement membrane material (Kamino bodies), and lack of ulceration [25]. |
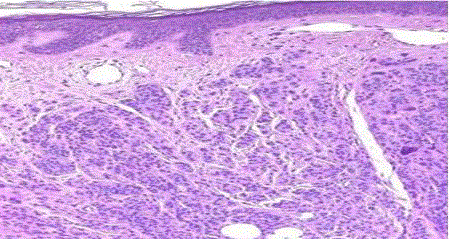
| Blue naevus and related nevus: Blue nevus of the ordinary type is usually small and located in the head, neck, or upper extremity. Microscopically, it is characterized by an ill-defined deep dermal proliferation of elongated and/or dendritic dermal melanocytes, sometimes extending into the subcutis. Melanin pigment is usually abundant (fig 11). |
| Blue nevi have also been reported in the sclera, hard palate, breast, vagina, cervix, prostate, spermatic cord, and lymph nodes. |
| The differential diagnosis of blue nevus includes dermatofibrosarcoma protuberans (if the melanin pigment is mistaken for hemosiderin) and – more importantly – metastatic melanoma from the skin or eye, which can simulate it closely [26]. |
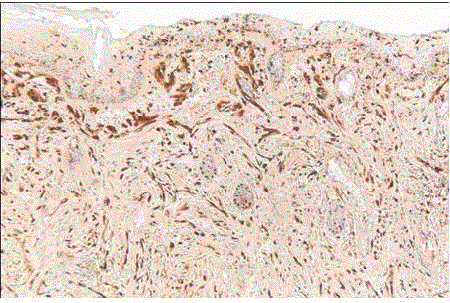
| They are positive for melanin stains, S-100 protein, and all other melanocytic stains, including HMB-45 [27] (fig 12). |
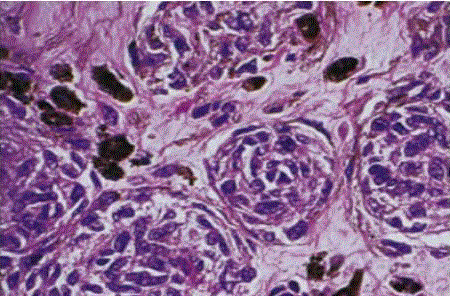
| Cellular blue nevus: is a distinctive variety of blue nevus that is often clinically suspected of being malignant because of its large size and intense pigmentation [28]. The most common locations are the buttock and sacrococcygeal areas, less common sites include the scalp, face, and dorsa of hands and feet. Microscopically, these lesions are extremely cellular, hence their name. Extension into the subcutis is the rule, and pigment can be easily found. They differ microscopically from malignant melanomas by the absence of junctional activity, no epidermal invasion, no peripheral inflammation, and no necrosis; there is presence of pushing margins, biphasic pattern, fasciculation, and neuroid structures. The nucleoli are inconspicuousness and there is relative lack of atypia and mitotic figures (fig 13). |
| Morphologic variants of cellular blue nevi can show occasional presence of a prominent cellular stroma between the tumor nests (desmoplastic cellular blue nevus),focal balloon cell change, paucity of pigmentation (amelanotic cellular blue nevus), and degenerative changes in the stroma, including pseudoangiomatous foci (ancient blue nevus). |
| Immunohistochemically, cellular blue nevi are positive for S-100 protein, Melan-A (fig 14), and HMB-45. Some congenital cellular blue nevi have also been found to be reactive for CD34 [29]. |
| Epithelioid Blue nevus: Microscopically, these nevi are heavily pigmented, poorly circumscribed dermal lesions composed of two types of melanocyte: one intensely pigmented and globular, and the other lightly pigmented, polygonal, and spindle [30]. |
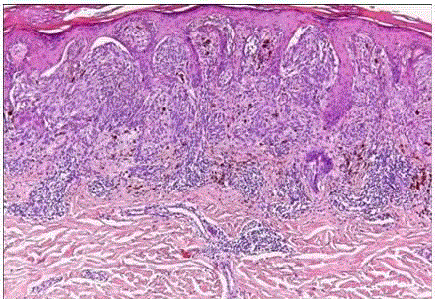
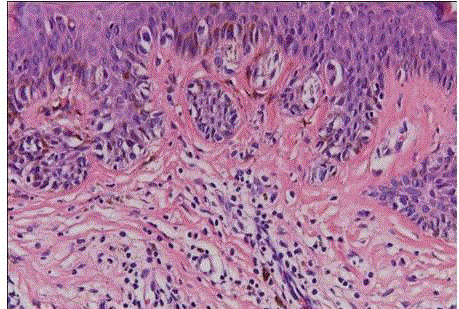
| Dysplastic nevi: it occurs as a genetically determined syndrome in families prone to develop malignant melanoma (dysplastic nevus syndrome). The nevi are clinically atypical, with a relatively large size (>5 mm), irregular outline, and variegated appearance. They appear in adolescence and continue to develop in adult life. Microscopically, most dysplastic nevi are compound nevi exhibiting marked lentiginous proliferation of melanocytes at the dermoepidermal junction, with or without theques. (fig 15) The latter are irregularly sized and shaped. These ques bridge adjacent rete ridges, which are themselves of irregular shape and orientation. If a dermal component is present, the junctional component is seen to extend beyond its lateral margins. The dermis shows eosinophilic and lamellar fibroplasia, focal perivascular lymphocytic infiltrate, and vascular dilation.These features, collectively referred to as architectural atypia, are usually matched by a mild to moderate degree of cytologicatypia, manifested by nuclear hyperchromasia, prominent nucleoli, and dusty melanin pigment. The melanocytes can be spindle shaped or epithelioid, the former tend to be arranged parallel to the skin surface [31]. |
| If the atypia is of a degree that indicatesmalignant neoplasm, we designate such lesion an in situ malignant melanoma arising in a compound nevus. For cases in which the atypia is below the threshold of melanoma (subjective determination), we use the termcompound nevus with atypia of the junctional component with the addition ‘(so-called dysplastic nevus)‘ if the above listed features for this ‘entity’ are present. |
| Malignant melanoma of the skin (melanoma) including specific histogenic types: The large majority of melanomas are associated with sunlight exposure and are thought to be due to ultraviolet radiation.[32] Therefore, most are found in the head and neck area and on the lower extremities ( in females) [33]. |
| Malignant melanomas can develop from nevi, but the exact percentage is not clear. It is obvious that only a small number of acquired nevi become malignant. The percentage is higher for congenital nevi and for dysplastic nevi, but the exact figures are again unknown. |
| There are four histogenic subtypes of Malignant melanoma. |
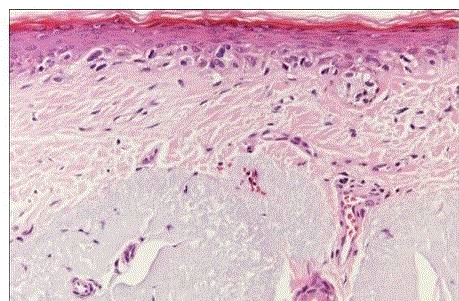
| Lentigomaligna melanoma: it typically occurs in the sun-exposed areas of elderly white persons, most commonly on the cheek [34]. It is a flat, slowly growing lesion, its color varying from tan to black.Microscopically, it is characterized by a proliferation of atypical melanocytes in the basal layer, distributed individually as well as in nests (fig 16). Retraction of the cytoplasm and pleomorphism are prominent [35]. |
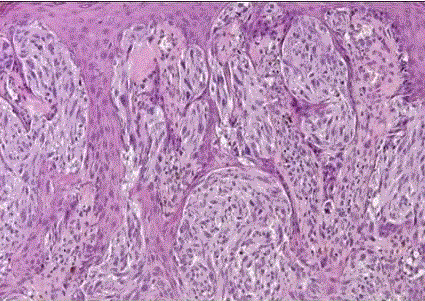
| Superficial spreading melanoma: it is the most common form of melanoma. It has also been called premalignant melanosis and pagetoid melanoma, and it can occur anywhere on the body surface. It has a variegated appearance, the colors including hues of tan, brown, black, blue, pink, and white. Microscopically, the noninvasive areas are composed of uniform atypical melanocytes with nest formation and pagetoid appearance (fig 17) |
| Nodular melanoma: it can present as a smooth nodule covered by normal epidermis, as an elevated blue–black plaque, or as a polypoid, frequently ulcerated mass [36] (fig 18). A lateral flat component is not seen clinically or microscopically. This type of melanoma affects all body surfaces and is usually of short duration. It occurs in a younger age group than either of the foregoing two categories. |
| (Acral) lentiginous melanoma: it is composed of an intraepidermal component of lentiginous type, which is similar in many respects to that seen in lentigomalina type [37] (fig 19). In contrast to the latter, however, the intraepidermal melanocytes tend to be dendritic and bizarre, the involved epidermis is markedly hyperplastic rather than atrophic, and the papillary dermis in this region is widened and inflamed. Tumors with these features have been seen on the palms, soles, subungual areas, mucocutaneous junction of the oral and nasal cavities, and anus. This type of melanoma is more common in black and Oriental people [38].
Typical malignant melanoma is easily identified microscopically because of its junctional activity, prominent melanin pigmentation, invasion of the surrounding tissue, marked cytologicatypia with nuclear grooves, folds, and pseudoinclusions, large eosinophilic nucleoli and abundant mitotic figures, some of them atypical [39]. |
| This is not always the case, as malignant melanoma shows great microscopic variability. The cells can be epithelioid, spindle shaped or extremely bizarre (‘monster cells’). Their size can range from small (lymphocyte-like) to that of giant multinucleated forms. The cytoplasm can be eosinophilic, basophilic, and foamy, of signet ring type, rhabdoid, oncocytic or completely clear (balloon cell melanoma).Melanin can be abundant, scanty, or absent (amelanotic melanoma) (fig 20) .Sometimes the amount of melanin production is so massive as to obscure the cellular details; such cases have been inelegantly called animal-type melanoma on the grounds that they resemble melanocytic neoplasms in horses and other mammals [40]. |
| The pattern of growth of melanoma may also show many variations. It can be pseudoglandular, pseudopapillary, peritheliomatous, hemangiopericytoma-like, resembling Spitz nevus (spitzoid melanoma), rosette-like, trabecular, verrucous (nevoid or pseudonevoid melanoma), carcinoid-like, or follicular, i.e., centered in the hair follicle and the adjacent dermis. The tumor can be accompanied by marked fibroblastic response, myxoid changes, osteoclast-like giant cells, Touton-like giant cells, pseudoepitheliomatous hyperplasia of the overlying epidermis, and a variety of divergent differentiations, includingosteo cartilaginous, rhabdomyoblastic, and neuroendocrine. Occasionally, formations suggesting differentiation toward Schwann cells, tactile corpuscles, ganglion cells, and other neuroid structures are observed. Sometimes the lymph node and other metastases of a melanoma acquire an appearance that is practically indistinguishable from that of a malignant peripheral nerve sheath tumor.[40] |
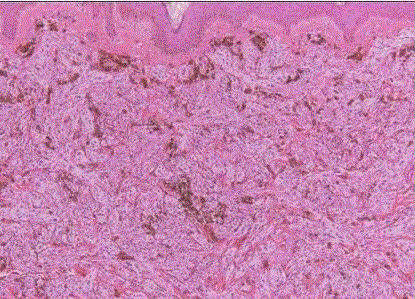
| Desmoplastic melanoma: it commonly affects the head and neck region [41]. This is an important subtype of melanoma because of its degree of aggressivity and the fact that it can be easily misdiagnosed, sometimes with serious consequences. Microscopically, it is formed of spindle cells surrounded by a heavy desmoplasticstroma. (fig 21) The tumor cells can be very scanty and not overly atypical. The difficulty is compounded by the fact that these cells are positive for S-100 protein but usually not for HMB-45, Melan-A, or any of the other more specific melanoma markers, while sometimes acquiring mesenchymal markers such as actin [42]. |
| Diagnosis at the H&E level are aided by focal fascicular pattern of growth, deep invasion, infiltration of nerves, round collections of lymphocytes at the tumor periphery (sometimes so prominent as to resemble cutaneous lymphoid hyperplasia/pseudolymphoma), and the presence of a lesion with the features of lentigomalinain the overlying epidermis [43]. But many of these features will often be absent in the individual case. Neurotropic melanomaisis a variant of desmoplastic/spindle cell melanoma that grows in a peripheral nerve sheath pattern. |
| Borderline or minimal deviation melanoma: is yet another proposed subtype of melanoma. It is a controversial concept that includes the previously cited verrucous and spitzoid melanoma, as well as a halo nevus-like type [44]. |
| Pigmented variant of Basal cell carcinoma: is also considered to be a melanocytic lesion. It is a cutaneous condition, a subtype of basal-cell carcinoma that exhibits increased melanization. About 80% of all basal cell carcinoma in Chinese are pigmented while this subtype is uncommon in white people. It is seen in areas commonly exposed to sunlight, usually on the neck, arms, face, head, hands and forearm. It is characterized by an irregularly pigmented well-marginated nodule with telangiectatic vessels on its surface. The centre may be depressed or ulcerated. It may sometimes appear as raw, dry skin on the chest and the tumor grows slowly in a course of months or years. Spread to other parts of the body is also rare. The color varies from light brown to dark black. The tumor may resemble malignant melanoma. Cancer cells in melanoma forms from the cells producing the skin pigments while this carcinoma affects the skin with its point of origin coming from the epidermis. |
| Benign lesions that are most commonly over diagnosed as melanomas are Spitz nevi (especially the desmoplastic type of pure epithelioid nevus), halo nevi, activated and dysplastic nevi, vulvar (genital-type) nevi, and nevi that have recurred following incomplete excision. |
| Conversely, the type of melanoma that is more likely to be underdiagnosed as benign is superficially spreading type, especially if occurring in sites such as the plantar and the subungual regions. The criteria listed here to be the most useful for the identification of early malignant melanoma. Unfortunately, none of them is pathognomonic. The diagnosis of melanoma can be made only on the basis of a combination of features [40]. |
| • Poor circumscription (lack of cohesiveness) of the intraepidermal melanocytic component. |
| • Lateral extension of individual melanocytes. Benign lesions usually exhibit a sharp cutoff, whereas in most malignant lesions there is a trailing off of atypical melanocytes spreading from the center of the lesion. |
| • Extension of melanocytes, individually and in nests, throughout the malpighian layer and within adnexal epithelium. This phenomenon of transepidermal migration results in a pagetoid appearance of the lesion, hence the term ‘pagetoid melanoma’ that is sometimes used. |
| • Size variation, shape variation, and confluence of melanocytic nests. |
| • Asymmetry. |
| • Lack of maturation of dermal melanocytes. |
| • Melanocytic atypia, evidenced by the prominence of nuclei and nucleoli and an increase of the nucleocytoplasmic ratio. |
| • Mitotic activity. Most benign nevi have a very small number of mitotic figures, although some spindle and epithelioid cell nevi may contain many. The presence of atypical mitoses is a strong indication that the lesion is malignant. |
| • Melanocytes with an abundant clear cytoplasm having a finely dispersed (’dusty’) chromatin. |
| • Necrosis of individual melanocytes. This phenomenon should be distinguished from the eosinophilic hyaline bodies (Kamino bodies) seen most often in Spitz nevi. |
| • Dermal infiltrate of chronic inflammatory cells, mainly lymphocytes. This is particularly prominent in early lesions and tends to have a bandlike distribution instead of the patchy appearance usually seen in irritated nevi. |
| Immunohistochemical stains— There is no single marker, or combination thereof, that establishes an unequivocal diagnosis of melanoma or nevus. Thus it is necessary to carefully analyze the pattern of expression (patchy versus diffuse) and localization (maturation) in context of morphological features. |
| Immunohistochemically, the typical melanoma is reactive for vimentin, S-100 protein, HMB-45, Melan-A, tyrosinase, and microphthalmia transcription factor. [45] Of these, vimentin is the most consistent (seen in practically 100% of the cases) but obviously the least useful diagnostically. Positivity for S-100 protein, although also nonspecific, is of greater practical importance because it is negative in many of the tumors that enter in the differential diagnosis. This reactivity is both nuclear and cytoplasmic, and is present in over 90% of the cases.It should be noted, however, that melanomas (particularly in their metastases) can lose their immunoreactivity for S-100 protein (and most of the markers discussed below) as a consequence of phenotypic dedifferentiation [46]. |
| HMB-45 is a much more specific marker than S-100 protein [47] (fig 12). It is particularly useful when the differential diagnosis includes a nonmelanocytic tumor that can also be S-100 protein positive, such as breast carcinoma. It may be detected in S-100 protein-negative melanomas, but on the whole it is less sensitive than the latter. It recognizes a premelanosomal glycoprotein (g100) related to the tyrosinase system, which explains why it may be negative in undifferentiated amelanotic neoplasms. Antibodies NK/1 beteb and HMB-50 recognize different epitopes on the same antigen. As already indicated, HMB-45 is generally negative in desmoplastic malignant melanoma. |
| Melan-A (Mart-1; A103) is a melanocyte differentiation antigen originally identified as a target for cytotoxic T cells, which is detected with the monoclonal antibody A103. It is positive in approximately 80% of melanomas and has become a widely used marker for this tumor (fig 14). However, it also stains steroid-producing cells from adrenal cortex, ovary, and testis, and the tumors originating from them [48]. |
| Tyrosinase is a key enzyme for the production of melanin from tyrosine; it can be detected with conventional enzyme histochemical methods (which require fresh tissue) but now also with immunohistochemistry or reverse transcriptase-polymerase chain reaction (RT-PCR) in paraffin-embedded material. At the immunohistochemical level, the positivity is in the range of over 90% [49]. Other tumors reacting to this marker are peripheral nerve sheath and neuroendocrine neoplasms. |
Stage IA: |
| • Lesions ≤ 1 mm in thickness with no evidence of ulceration or metastases (T1aN0M0) are associated with a 5-y survival rate of 95% |
| Stage IB: |
| • Lesions ≤ 1 mm in thickness with ulceration noted but without lymph node involvement (T1bN0M0) or lesions 1.01-2 mm in thickness without ulceration or lymph node involvement (T2aN0M0) are associated with a 5-y survival rate of approximately 91% |
Stage IIA: |
| • Melanomas > 1 mm but ≤ 2 mm in thickness with no evidence of metastases but with evidence of ulceration (T2bN0M0) or lesions 2.01-4.0 mm in thickness without ulceration or lymph node involvement (T3aN0M0) are associated with an overall 5-y survival rate of 77-79% |
Stage IIB: |
| • Melanomas 2.01-4 mm in thickness with ulceration but no lymph node involvement (T3bN0M0) or lesions > 4 mm in thickness without ulceration or lymph node involvement (T4aN0M0) are associated with a 5-y survival rate of 63-67% |
Stage IIC: |
| • Lesions > 4 mm in thickness with ulceration but no lymph node involvement (T4bN0M0) are associated with a 5-y survival rate of 45% |
Stage IIIA: |
| • Patients with any-depth lesion, no ulceration, and 1 positive (micrometastatic) lymph node (T1-4aN1aM0) have a 5-y survival rate of 70% |
| • T1-4aN2aM0 lesions (any-depth lesion, no ulceration, but 2-3 nodes positive for micrometastasis) are associated with a 5-y survival rate of 63% |
Stage IIIB: |
| • Patients with any-depth lesion, positive ulceration, and 1 lymph node positive for micrometastasis (T1-4bN1aM0) or 2-3 nodes positive for micrometastasis (T1-4bN2aM0) have a 5-y survival rate of 50-53% |
| • Patients with any-depth lesion, no ulceration, and 1 lymph node positive for macrometastasis (T1-4a, N1b, M0) or 2-3 nodes positive for macrometastasis (T1-4aN2bM0) have a 5-y survival rate of 46-59% |
Stage IIIC: |
| • Patients with any-depth lesion, positive ulceration, and 1 lymph node positive for macrometastasis (T1-4bN1bM0); 2-3 nodes positive for macrometastasis (T1-4bN2bM0); or ≥ 4 metastatic lymph nodes, matted lymph nodes, or in-transit met(s)/satellite(s) have a 5-y survival rate of 24-29% |
Stage IV: |
| • Melanoma metastatic to skin, subcutaneous tissue, or lymph nodes with normal LDH level (M1a) is associated with a 5-y survival rate of 19% |
| • M1b disease (metastatic disease to lungs with normal LDH level) has a 5-y survival rate of 7% |
| • M1c disease (metastatic disease to all other visceral organs and normal LDH level or any distant disease with elevated LDH level) is associated with a 5-y survival rate of 10% |
Conclusion |
| Though it is a challenge for a histopathologist to make a specific diagnosis of a case of melanocytic tumor but with clinical correlation and step by step approach aided with IHC, most of the tumors can be diagnosed correctly. Staging of the tumors is obviously done after proper consultation with dermatologist. |
References |
- Boyle JL, Haupt HM, Stern JB, Multhaupt HAB. Tyrosinase expression in malignant melanoma, desmoplastic melanoma, and peripheral nerve tumors: an immunohistochemical study. Arch Pathol Lab Med. 2002;126:816-822.
- Ramsay JA, From L, Kahn HJ. bcl -2 protein expression in melanocytic neoplasms of the skin. Mod Pathol. 1995;8:150-154.
- Rook, Arthur. "lentigos, melanocytic nevi and melanoma." In Rook's textbook of dermatology, 54.1. 8th ed. Chichester, West Sussex, UK: Wiley-Blackwell, 2010.
- Silvers DN, Helwig EB: Melanocytic nevi in neonates. J Am AcadDermatol. 1981;4:166-175.
- James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier.
- Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. pp. 1736âÃâ¬Ãâ8.
- Sowa J, Kobayashi H, Ishii M, Kimura T. Histopathologic findings in UnnaâÃâ¬Ãâ¢s nevus suggest it is a tardive congenital nevus. Am J Dermatopathol. 2008;30:561-566.
- Gardner WA Jr, Vazquez MD. Balloon cell melanoma. Arch Pathol. 1970;89:470-472.
- Wayte DM, Helwig EB: Halo nevi. Cancer. 1968;22:69-90.
- Guzzo C, Johnson B, Honig P. Cockarde nevus. A case report and review of the literature. PediatrDermatol. 1988;4:250-253.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. A frequent participant in combined nevus. J CutanPathol. 1992;19:172-180.
- Cesinaro AM, Foroni M, Sighinolfi P, Migaldi M, Trentini GP. Spitz nevus is relatively frequent in adults: a clinico-pathologic study of 247 cases related to patientâÃâ¬Ãâ¢s age. Am J Dermatopathol. 2005;27:469-475.
- Mooi WJ. Spitz nevus and its histologic simulators. AdvAnatPathol. 2002;9:209-221.
- Tu P, Miyauchi S, Miki Y. Proliferative activities in Spitz nevus compared with melanocytic nevus and malignant melanoma using expression of PCNA/cyclin and mitotic rate. Am J Dermatopathol. 1993;5:311-314.
- Sagebiel RW, Chinn EK, Egbert BM. Pigmented spindle cell nevus. Clinical and histologic review of 90 cases. Am J Surg Pathol. 1984;8:645-653.
- Barr RJ, Morales RV, Graham JH. Desmoplastic nevus. A distinct histologic variant of mixed spindle cell and epithelioid cell nevus. Cancer. 1980;46:557-564.
- PaniagoPereira C, Maize JC, Ackerman AB. Nevus of large spindle and/or epithelioid cells (SpitzâÃâ¬Ãâ¢s nevus). Arch Dermatol. 1978;114:1811-1823.
- Busam KJ, Barnhill RL. Pagetoidspitz nevus. Intraepidermal Spitz tumor with prominent pagetoid spread. Am J Surg Pathol. 1995;19:1061-1067.
- Howat AJ, Variend S. Lymphatic invasion in Spitz nevi. Am J Surg Pathol. 1985;9:125-128.
- Scott G, Chen KTK, Rosai J. Pseudoepitheliomatus hyperplasia in SpitzâÃâ¬Ãâ¢s nevi. A possible source of confusion with squamous cell carcinoma. Arch Pathol Lab Med. 1989;113:61-63.
- Burg G, Kempf W, Hochil M, Huwyler T, Panizzon RG. âÃâ¬ÃËTubularâÃâ¬Ã⢠epithelioid cell nevus: a new variant of SpitzâÃâ¬Ãâ¢s nevus. J CutanPathol. 1999;25:475-478.
- Clarke B, Essa A, Chetty R. Plexiformspitz nevus. Int J SurgPathol. 2002;10:69-73.
- Harvell JD, Meehan SA, LeBoit PE: SpitzâÃâ¬Ãâ¢s nevi with halo reaction: a histopathologic study of 17 cases. J CutanPathol. 1998;24:611-619.
- Diaz-Cascajo C, Borghi S, Weyers W. Angiomatoid Spitz nevus: a distinct variant of desmoplastic Spitz nevus with prominent vasculature. Am J Dermatopathol. 2000;22:135-139.
- Walsh N, Crotty K, Palmer A, McCarthy S. Spitz nevus versus spitzoid malignant melanoma: an evaluation of the current distinguishing histopathologic criteria. Hum Pathol. 1998;29:1105-1112.
- Wieselthier JS, White WL. Cutaneous metastasis of ocular malignant melanoma: an unusual presentation simulating blue nevi. Am J Dermatopathol. 1996;18:289-295.
- Sun J, Morton TH, Gown AM. Antibody HMB-45 identifies the cells of blue nevi. An immunohistochemical study on paraffin sections. Am J Surg Pathol. 1990;14:748-751.
- Masson P. Neuro-nevi âÃâ¬ÃËbleuâÃâ¬Ãâ¢. Arch De VecchiAnat Patol.14:1-28. 1950
- Smith KJ, Germain M, Williams J, Skelton H. CD34-positive cellular blue nevi. J CutanPathol. 2001;28:145-150.
- Carney JA, Ferreiro JA. The epithelioid blue nevus: a multicentric familial tumor with important associations, including cardiac myxoma and psammomatousmelanoticschwannoma. Am J Surg Pathol. 1997;20:259-272.
- Barnhill RL, Roush GC, Duray PH. Correlation of histologic architectural and cytoplasmic features with nuclear atypia in atypical (dysplastic) nevomelanocytic nevi. Hum Pathol. 1990;21:51-58.
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation.N Engl J Med. 1999;340:1341-1348.
- Gussack GS, Reintgen D, Cox E, Fisher SR, Cole TB,Seigler HF. Cutaneous melanoma of the head and neck. A review of 399 cases. Arch Otolaryngol. 1983;109:803-808.
- McGovern VJ. The nature of melanoma. A critical review. J CutanPathol. 1982;9:61-81.
- Clark WH Jr, Mihm MC Jr: Lentigomaligna and lentigo-maligna melanoma. Am J Pathol. 1969;55:39-67.
- Manci EA, Balch CM, Murad TM, Soong S-J. Polypoid melanoma, a virulent variant of the nodular growth pattern. Am J Clin Pathol. 1981;75:810-815.
- Krementz ET, Reed RJ, Coleman WP III, Sutherland CM, Carter RD, Campbell M. Acrallentiginous melanoma. A clinicopathologic entity. Ann Surg. 1982;195:632-645.
- Kwon IH, Lee JH, Cho KH. Acrallentiginous melanoma in situ: a study of nine cases. Am J Dermatopathol. 2004;26:285-289.
- Barnhill R, Mihm M. The histopathology of cutaneous malignant melanoma. SeminDiagnPathol. 1993;10:47-75.
- https://www.inkling.com/read/rosai-and-ackermans-surgical-pathology-rosai-10th/chapter-4/melanocytes .
- Kay PA, Pinheiro AD, Lohse CM, Pankratz VS, OlsenKD, Lewis JE, Nascimento AG. Desmoplastic melanoma of the head and neck: histopathologic and immunohistochemical study of 28 cases. Int J Surg Pathol. 2004;12:17-24.
- Kucher C, Zhang PJ, Pasha T, Elenitsas R, Wu H, Ming ME, Elder DE, Xu X. Expression of Melan-A and Ki-67 in desmoplastic melanoma and desmoplastic nevi. Am J Dermatopathol. 2004;26:452-457.
- Kaneishi NK, Cockerell CJ. Histologic differentiation of desmoplastic melanoma from cicatrices. Am J Dermatopathol. 1998;20:128-134.
- Boyle JL, Haupt HM, Stern JB, Multhaupt HAB. Tyrosinase expression in malignant melanoma, desmoplastic melanoma, and peripheral nerve tumors: an immunohistochemical study. Arch Pathol Lab Med. 2002;126:816-822.
- deWit NJ, van Muijen GN, Ruiter DJ. Immunohistochemistry in melanocytic proliferative lesions. Histopathol. 2004;44:517-541.
- Aisner DL, Maker A, Rosenberg SA, Berman DM. Loss of S100 antigenicity in metastatic melanoma. Hum Pathol.36:1016-1019. 2005
- Bacchi CE, Bonetti F, Pea M, Martignoni G, Gown AM. HMB-45:a review. ApplImmunohistochem. 1996;4:73-85.
- Jungbluth AA, Busam KJ, Gerald WL, StockertE,Coplan KA, Iversen K, MacGregor DP, Old LJ,
- Chen YT. A103: an anti-Melan-A monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol. 1998;22:595-602.
- Hofbauer GF, Kamarashev J, Geertsen R, Boni R, Dummer R. Tyrosinaseimmunoreactivity in formalin-fixed, paraffin-embedded primary and metastatic melanoma: frequency and distribution. J CutanPathol. 1998;25:204-209.
- NCCN Clinical Practice Guidelines in Oncology: Melanoma. V.3. 2011;Accessed March 28, 2011. Available at http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. Melanoma of the skin. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
|