ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
M.S.Anish Fathima1, Mrs.K.Latha2 and Dr.B.Umamaheshwari1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
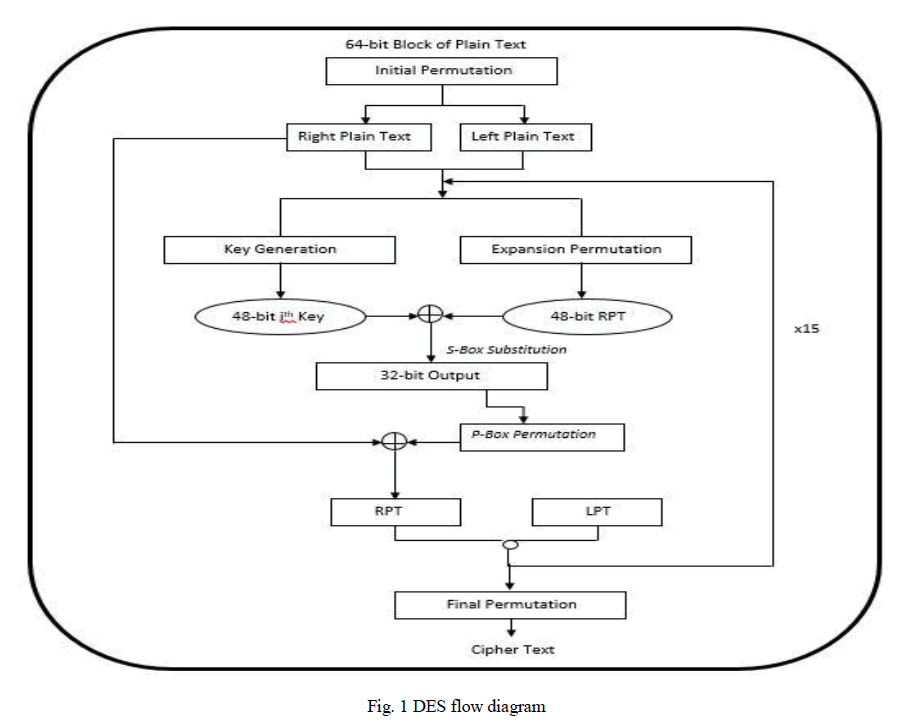
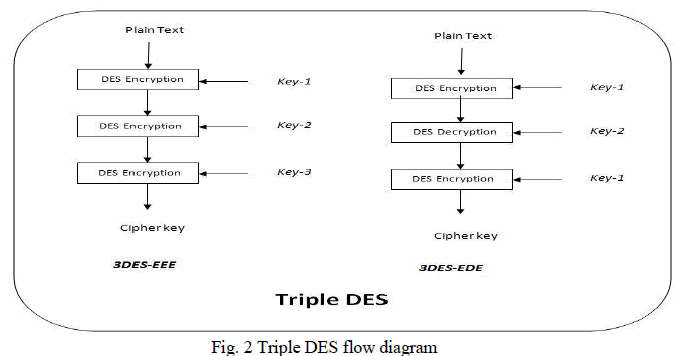
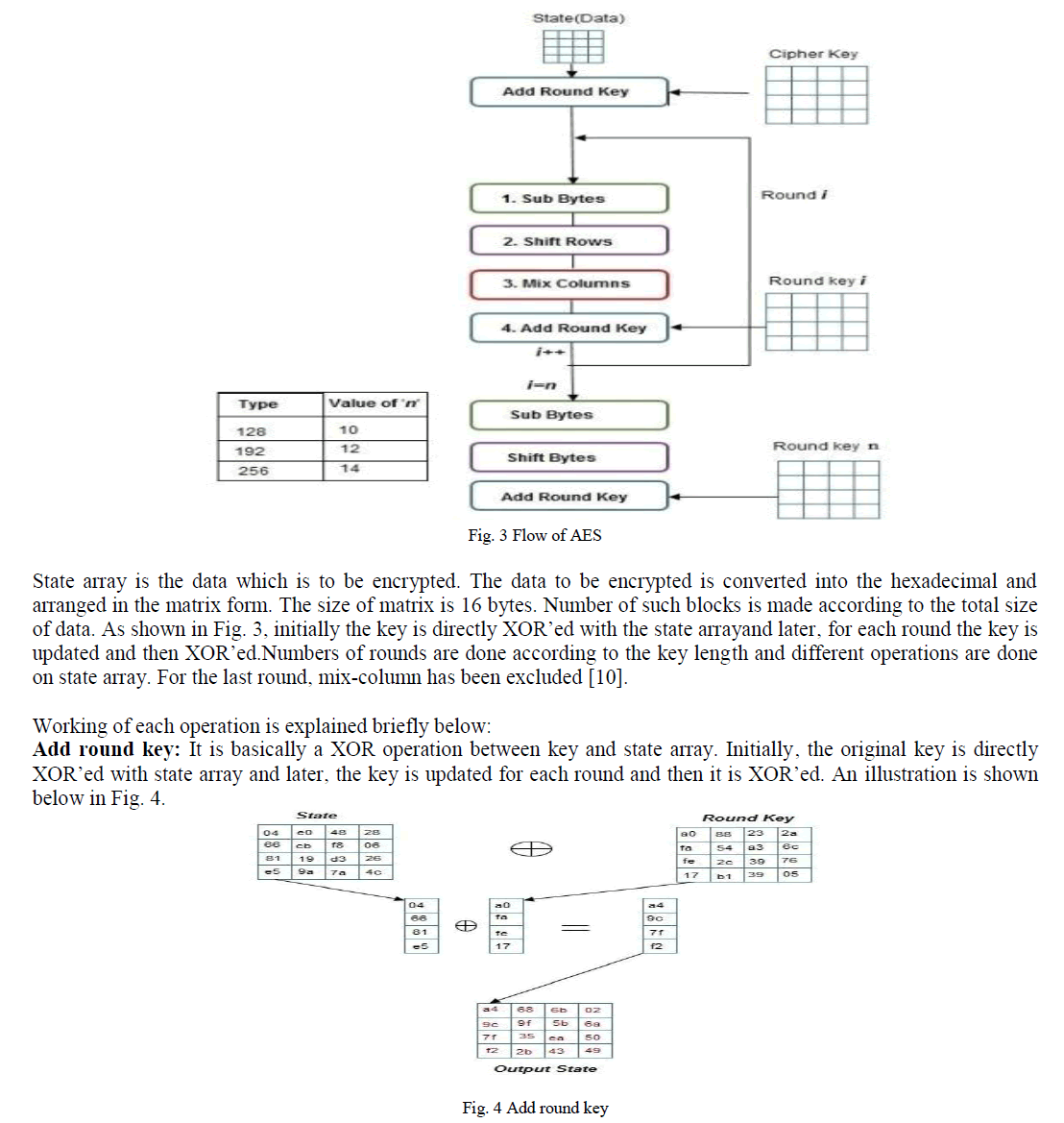
Proton Exchange membrane fuel cells (PEMFC) have been received increasing attention from both the public and fuel cell community due to their great potential for transport applications. It has been widely considered as one of the most promising clean power sources because of its capability for zero-emission operation with high efficiency. In this paper, a compact model has been developed for Multi-cells in a Proton Exchange Membrane fuel cell stack by using MATLAB/SIMULINK. Activation, Ohmic and Concentration losses are also considered to obtain better characteristics of fuel cell. This paper explicates how the fuel flow rate of a single fuel cell affects the stack voltage. Also this paper clearly shows that the membrane thickness of a cell affects the stack voltage in negative way. It has been shown through transient responses .This Multi-cell model will be useful for fault diagnosis methods.
Keywords |
| Proton Exchange Membrane Fuel cell, Membrane thickness, Multi-cells, PEMFC. |
INTRODUCTION |
| Now a day the generation of power sources is an important one. All technologies are focused to generate the power. Fuel cells are considered to be the most efficient alternative energy conversion devices. Fuel cells generate electricity by an electrochemical reaction in which oxygen and a hydrogen-rich fuel combine to form water. Unlike internal combustion engines, the fuel is not combusted, the energy instead being released electro catalytically. This allows fuel cells to be highly energy efficient, especially if the heat produced by the reaction is also harnessed for space heating, hot water or to drive refrigeration cycles. Fuel cells are classified many types according to their operating temperature. |
| Among that Proton Exchange Membrane Fuel Cell (PEMFC) is considered as one of the most promising clean power sources because of its capability for zeroemission operation with high efficiency. The proton exchange membrane fuel cell (PEMFC) uses a waterbased, acidic polymer membrane as its electrolyte, with platinum-based electrodes. PEMFC cells operate at relatively low temperatures (below 100 degrees Celsius) and can tailor electrical output to meet dynamic power requirements. Due to the relatively low temperatures and the use of precious metal-based electrodes, these cells must operate on pure hydrogen. PEMFC cells are currently the leading technology for light duty vehicles and materials handling vehicles, and to a lesser extent for stationary and other applications. The PEMFC fuel cell is also sometimes called a polymer electrolyte membrane fuel cell (also PEMFC). |
| At anode side the catalyst ionizes hydrogen as H+ ions and electrons [1], |
 |
| Figure 1. Operation principle of Fuel Cell |
| The V-I characteristics of PEMFC can be illustrated by polarization curve in Fig 2. This polarization curve can be divided into three regions , Activation overpotential, Ohmic overpotential, Concentration overpotential [3]. In Activation overpotential region, the dominant source of losses is due to the slowness of the chemical reactions. Ohmic loss occurs due to the internal resistance. Concentration loss occurs due to mass transport limitations are dominant.Concentration overpotential occurs when the chemical reaction is limited by the rate at which reactants can be supplied. |
 |
| It is the goal of the model developed here to enhance the performance of the fuel cell stack and one of the criteria for degradation of cell voltage has been found. |
| The voltage of a single fuel cell is quite small , about 0.7V [1] when drawing useful current, This means that to produce a useful voltage many cells have to be connected in series. Such a collection of fuel cells in series is known as a „StackâÃâ¬ÃŸ. The most obvious way to do this is by simply connecting the edge of each anode to the cathode of the next cell. |
| To improve the voltage cells are connected as a „StackâÃâ¬ÃŸ. If 10 cells are connected in serious it should produce nearly 7V , but practically it is not possible. The contribution of this paper is that what are all the reasons behind that voltage degradation . In this paper 10 cells, 5 cells are individually connected and the steady state and dynamic performance have been discussed. Many models are available for fuel cell but this model is quite simple and it will be very useful to detect the fault cell in a fuel cell stack. |
| This paper represents that the Steady state and Dynamic characteristics of Multiple cells in a Proton Exchange Membrane fuel Cell stack. It shows how the stack performance varies with the variation of fuel flowrate of a single cell. Also how the change in membrane thickness of a single cell affects the stack voltage |
 |
| The Dynamic Equations are, |
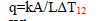 |
RESULTS AND DISCUSSION |
| A. Causes of Water Flooding |
| Water flooding play an important role in the performance of Proton Exchange membrane Fuel cell. Some main reasons for this water flooding are, Due to the “Back-Diffusion” some water molecules are diffused from cathode to anode [8]. The another reason, water will be dragged from anode to cathode by protons moving through the electrolyte.,the another reason ,Due to the Humidification of Fuel and Oxygen. Accumulation of excess water limits the fuel flow rate therefore the performance decreases. During this flooding event Membrane water content increases thus membrane thickness increases. |
| B. Causes of change in Membrane Thickness |
| One of the main reason for change in membrane thickness is water flooding, due to this water flooding the water content in membrane will increase. The membrane thickness has negative effect on the performance of the cell because as the thickness increases, the moving time of proton from anode to cathode increases [9], hence it delays the reaction on the cathode side which results in a stack voltage. |
| C. Steady state Responses |
| The Fig 3. Shows that the effect of membrane thickness on cell voltage. For a particular current performance of individual cells is plotted. 10 cells are connected individually and the membrane thickness of two cells are varied with other cells for a current 18 A. |
 |
| D. Transient Responses |
| In this dynamic model 5 cells are connected individually 5lpm of fuel flow rate and 3lpm of air flow rate have been supplied for 4 cells . The second cell only get 1lpm of fuel flow due to some pressuredrop. The Fig 4. clearly shows how the fuel flow rate affects cell voltage. |
 |
 |
CONCLUSION |
| In this paper Dynamic model has been developed for Muliple cells of Proton Exchange Membrane Fuel cell stack by using MATLAB/SIMULINK Software .The steady state performance of multiple cells also discussed. This paper shows that how the membrane thickness of a single cell affects the stack voltage . The reasons behind that change of membrane thickness are also discussed. The further progress is going on CFCT. In Future ,this model will be useful for fault detection techniques. |
ACKNOWLEDGMENT |
| The authors would like to express sincere thanks to Dr.N.RAJALAKSHMI, Senior Scientist, Centre for Fuel Cell Technology, Chennai, for her support to do this work. |
References |
|