ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Richa Srivastava
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
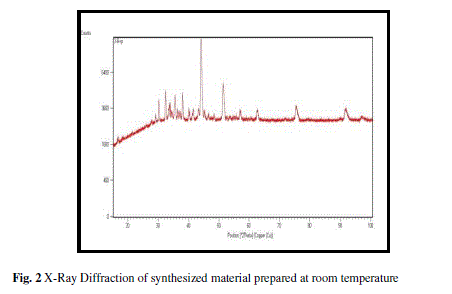
Present paper explores the synthesis of nanocomposite NiFe2O4-Fe2O3-NiO and its relevance as liquefied petroleum gas sensor. The as prepared material was characterized using Scanning electron microscope and X-ray diffractometer. The X-ray diffraction reveals the formation of nickel ferrite along with ferric and nickel oxides. The average crystallite size of material was found to be 4.62 nm. SEM images exhibit the porous nature of sensing material with a number of active sites. Thick film of material was fabricated using screen printing techniques and was investigated with the exposition of liquefied petroleum gas. Variations in resistence of thefilm withtime fordifferent concentration of LPG were recordedat room rempereture. The maximum value of sensitivity was found 5733 for 4 vol.% of LPG. These experimental results show that synthesized nanocomposite material is a promising material for LPG sensor.
Keywords |
| Nanomaterial, sensitivity, morphology, LPG |
I. INTRODUCTION |
| Spinel ferrites, with common formula of MFe2O4 (M: a divalent metal ion), have wide technological applications, e.g., in multilayer chip inductor (MLCI), ferrofluids, high-speed digital tape or recording disks, rod antenna, and humidity sensor [1-4]. Generally, for magnetic or electrical applications, ferrites with high-density are to be used. But, there are many applications, such as gas or humidity sensors, for which lower density and nanosized structures are preferred. As suggested by several authors [5-6] nanosized grains of sensing materials are preferred to increase the specific surface exposed to gas. The ferrites have demonstrated to be good materials for semiconductor gas and humidity sensors [7-8]. Nickel ferrite is considered to be the most promising highly sensing materials of sensors due to the temperature dependent surface morphology and photo catalytic activity [9]. NiO is also various applications as sensors [10-12]. |
II. EXPERIMENTAL |
| A. Synthesis of material |
| NiFe2O4-Fe2O3-NiO composite material has been synthesized by conventional precipitation method by stoichiometric amount of [Ni(CH3COO)2 .2H2O] and Fe( NO3)3 were separately dissolved in isopropyl alcohol and sodium hydroxide (NaOH) solution was added drop by drop to obtain NiFe2O4 in hydroxide form. Poly Ethylene Glycol-400 (PEG-400) was added as capping reagent. Vigorous stirring was carried out for 20-24 h to ensure complete and intimate reaction between various components. Later it was dried for 8-10 h at 100°C in an oven and further calcined at 400°C for 2 h, which results a complete crystallization into powder. |
| A. Fabrications of Thick Films |
| Thick Films of sample was prepared by screen printing method on taking alumina as substrate. The synthesized powder was dissolved in isopropyl alcohol and these were sonicated for 15-20 min. The sonicated solutions were stirred at 80ïÃâðC for 6 h. The obtained paste was used for fabrication of thick films The paste was screen printed on alumina substrate. The film was drying at 120ïÃâðC for 4 h. This drying procedure stabilizes the film. Further the film was annealed at 400ïÃâðC which converts the films as sensing materials. Silver contacts on two opposite ends of the film were made. Sensing film with silver contacts was used for gas sensing measurements. |
| B. Characterizations of Material |
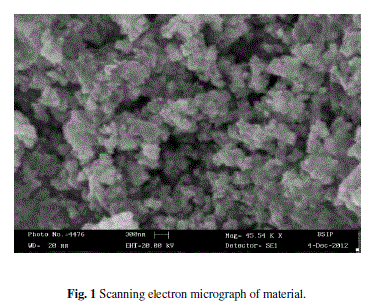
| The surface morphology of the synthesized powder in form of the thick film and films was analysed using a scanning electron microscope (SEM, LEO-Cambridge). Figure 1, scanning electron micrograph exhibits that thick film fabricated from material shows more active sites, giving largest effective surface area. This enables larger surface area for adsorption of gas giving more response. |
 |
 |
| C. Gas Sensing Measurements |
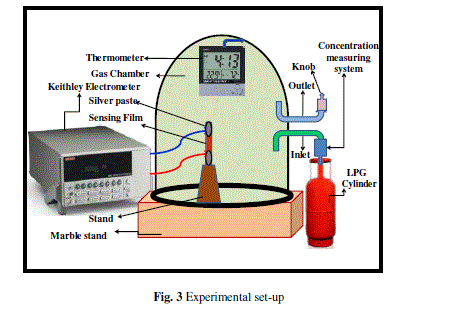
| Prima facie before the exposition of LPG to the sensing element, the gas chamber was allowed to evacuate at room temperature for 15–20 min and the stabilized resistance was taken as Ra. For the LPG sensing measurements a special gas chamber was designed which consists of a gas inlet and an outlet knob for LPG exclusion. The schematic diagram of LPG sensing set-up is shown in Figure 3. The sensing film was inserted between the two Ag electrodes inside the glass chamber having two knobs. One knob is associated with the concentration measuring system (gas inlet) and other is an outlet knob for releasing of the gas. Now this was exposed with LPG and variations in resistance with the time for different vol % of LPG were recorded by using Keithley electrometer (Model: 6514) |
 |
| Sensitivity of the LPG sensor is defined as the change in resistance in the presence of gas (Rg) to the resistance in presence of air (Ra) that is |
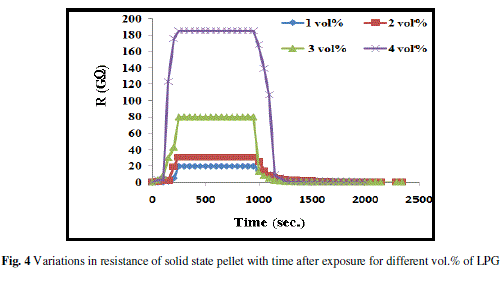
| Fig. 4 illustrates the variations in resistance of the film with time at different vol.% of LPG at room temperature. Curve for 1 vol.% and for 2 vol.% of LPG show small variation in resistance with time after exposure. Curve for 3 vol.% of LPG exhibits improved response and has better sensitivity than 1 and 2 vol.% both. Further, for 4 vol.% of LPG, the resistance increases sharply with time after exposure up to 250 s and then become constant. Fig. 5 exhibits the variations of average sensitivity with different concentrations of LPG and it was found that as the concentration of LPG (vol.%) increases, the average sensitivity of sensor increases slowly upto 3 vol.% of LPG, later it increases rapidly. The maximum sensitivity was obtained for 4 vol.% of LPG and is ~ 5733. |
 |
III. GAS SENSING MECHANISM |
| The LPG sensing mechanism is based on the changes in the resistance of the film. The oxygen adsorbed on the surface of the film influences the resistance of the NiFe2O4-Fe2O3-NiO composite material based sensor. Initially oxygen from the atmosphere adsorbs on the surface of the film and extracts electrons from its conduction bands to form O2 - species on the surface, consequently resistance increases. After that an equilibrium state is achieved between oxygen of material and atmospheric oxygen and the value of stabilized resistance was found. When the film is exposed to LPG, it reacts with the chemisorbed oxygen. On interaction with hydrocarbons (CnH2n+2) of LPG the adsorbed oxygen is removed, forming gaseous species and water vapor. Consequently, the resistance changes, which is due to the change in the width of depletion layer after exposure to LPG. |
| When the LPG reacts with the surface oxygen ions then the combustion products such as water depart and a potential barrier to charge transport would be developed i.e., this mechanism involves the displacement of adsorbed oxygen species by formation of water. The formation of barrier is due to reduction in the concentration of conduction carriers and thereby, results in an increase in resistance of the sensing element with time. As the pressure of the gas inside the chamber increases, the rate of the formation of such product increases and potential barrier to charge transport becomes strong which has stopped the further formation of water constituting the resistance constant. |
IV. CONCLUSION |
| We have successfully synthesized NiFe2O4-Fe2O3-NiO composite material having average crystallite size 4.62 nm via chemical precipitation method and fabricated its thick film by using screen printing technology. The screen printing method has certain advantages such as low cost, simple in construction and having excellent sensing properties. We have investigated the behaviour of the gas sensitivity as a function of gas concentration. It was found that synthesized material works as a good LPG sensor at room temperature and maximum sensitivity of this sensor was found ~ 5733 for 4 vol. % LPG. Good sensitivity and stability demonstrate the promise of fabricated thick film of material for LPG detection in the industrial and environment monitoring |
ACKNOWLEDGEMENT |
 |
References |
 |