ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
F. O. Faithpraise1, J.Idung2, B. Usibe3, C. R. Chatwin4, R.Young5, P.Birch6
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The main impact of mosquito pests is the transmission of many dangerous diseases and death. Hence, the reduction of their population by the use of a natural control method is a primary objective of this research. This mosquito reduction method utilises different species of predators (Odonata) and (Toxorhynchites) to substantially improve the environment. The frequency of capturing the pest mosquitoes by the predators is determined using a Pascal distribution, whilst insect mortality is modelled using a Weibull distribution. The results from the model show that by using insect predators, a significant reduction of the mosquito population is possible in less than eighty days.
Keywords |
| Natural control; Mosquito control; Odonata; Toxorhynchites; Disease Vector Control |
INTRODUCTION |
| Several species of mosquitoes are a public health nuisance for the world and Africa as a continent as they drive extremely dangerous disease vectors. In order to manage public health, it is important to understand the lifecycle of the dangerous mosquito species and the agents that can control them. |
Mosquito Lifecycle |
| All mosquito pest go through complete metamorphoses, from: egg to larva to pupa to adult, see figure 1. Their cycles are identical with slight differences and preferences relating to water clarity. The cycle begins when a female mosquito obtains a blood meal from either a human-being or other mammal to supply the required nutrients to produce as many as two hundred and fifty eggs at a time. She then seeks an aquatic location usually on the surface of stagnant water, or in a water filled depression, or on the edge of a container, where rainwater may have collected to lay eggs. After two days, the eggs hatch into larvae. The larvae live and feed on microorganisms in the water for seven to fourteen days and develop into pupae, which no longer feed. The mosquito then emerges from the pupa shell as a fully developed adult after four days. The moment the body of the mosquito finishes moulting, it is hypersensitive to carbon dioxide exhaled from mammals, it has poor eyesight and is very sensitive to mammal sweat scent from a half mile distance [1], [2]; this enables it to locate a mammal target to seek out a blood meal by a bite on the animal or human skin. As the mosquito punctures the skin it injects its saliva into the flesh. If the mosquito is infected with any kind of disease, it is transferred into the prey through the saliva. There are about 2500 species of mosquitoes on the planet, of which 300 are well known disease carriers. Different species carry different diseases that are local to the area where they live; mosquitoes can attack and breed at different times of the day. Worldwide approximately seventy million people per annum catch diseases from mosquito bites [3].Several attempts to manage and control the population of the harmful mosquito species have failed. Most of these dangerous species can be classified into: Permanent Water Mosquitoes, Floodwater Mosquitoes and Tree Hole/artificial container Mosquitoes. Permanent Flood Water Mosquitoes are found in swamps, ponds, lagoons, and ditches that permanently have water all year around, they include:Aedesas illustrated by [4],[5], [6], [7]; Culexpipiens,[8]; Culicinae,[9]; Culiseta,[10]and Anopheles gambiae[11]. Floodwater Mosquitoes are found in areas that are flooded temporarily or seasonally and have the fastest breeding cycle of all mosquitoes up to 7 days. Tree hole/artificial container Mosquitoes are found and breed in: empty buckets, old tires, and anything that can hold water. Research has confirmed that these species of mosquitoes are epidemiologically a major vector for the spread of infectious disease,[12]; yellow fever virus,[13]; dengus fever,[14], [15]; Chikungunya fever,[16], [17]; Japanese encephalitis[18], [19]; Meningitis[20], [21]; Urticaria [22], [23]; West Nile virus [24], [25]and Dirofilariaimmitis [26]. |
RELATED WORK |
| Most notable attempts made to control and manage the mosquito population and the deleterious effect of some classes of mosquitoes includes the design of an adhesive film trap to catch the egg depositing mosquitoes by Facchinelli, et al[27], and Gama, et al [28].Thecomparison of the catching effect of gold standard traps with other traps was done by [29], and observed that for the trapping system to be successful users must comply with the deployment instructions, otherwise the trapping method is rendered ineffective with a corresponding increase in mosquito population density. [30], [31] implemented successful control of some species of mosquitoes using Bacillus sphaericusbut Bacillus sphaericus usage could not continue due to its long term side effects on humans and some arthropods [32].[33]evaluated the control of mosquitoes with Romanomermisyunanensis(Nematoda: Mermithidae). [ 34]demonstrated thesubstantial controls of the Culex mosquitoes larvae by the used of fish muddy loaches (Misgurnusmizolepis), but the adult mosquitoes were not controlled. [35] proposed the combination of some chemical component like imidacloprid and permethrin. [36] proposed the use of entomopathogenic fungus for the control of adult mosquito. [37] proposed the use of predator (Odonata) and its life cycle stages. Because of the long term harmful effect of most of the chemical pesticides and the increased resistance of mosquitoes [38], more favourable and better methods of mosquito control are still under research. [39],[40]suggested and demonstrated programmes based on environmental modification of pond and pool filling and several preventive measures based on mosquito breeding habitats as follows: |
| • Empty water from containers such as flower pots, birdbaths, pet water dishes, cans, gutters, tires and buckets regularly to disrupt the mosquito breeding cycle. |
| • Keep windows and door screens in good working order to prevent mosquitoes from entering domiciles. |
| • Put on long-sleeved cloths while outdoors, consider staying indoors early in the morning and evening when mosquitoes are most active. |
| • Use mosquito netting both in and outdoors. |
| • Consider using an insect repellent. |
| • Keep gutters clean and unclogged. |
| • Walk properties after rainfall and be sure downspouts are drained properly, without leaving puddles in the drainage area. |
| • Keep swimming pools cleaned and chlorinated, even when not in use to prevent mosquito breeding. |
| • Ornamental ponds should be aerated to keep water moving and discourage mosquitoes from laying eggs. Alternatively, stock the pond with mosquito-eating fish. |
| This kind of programme is important but requires considerable time and financial investment, it does rely on people being very active, which is often difficult to sustain. |
| Temporary mosquito control methods |
| Some temporary mosquito control methods available on the market today include: Mosquito candles, [41], [42], [43]. Citronella candles are made from the essential oils of citronella grass with natural mosquito repellents. While the fragrance may deter mosquitoes, it will still not prevent the bite once the plume of the fragrance the candle releases expire. Conceal candles was made available as an improvement which delivers an inhibitor molecule found in the essential oils of certain plants to render the mosquitoes incapable of smelling and thereby incapable of finding someone to bite. However this product still has short comings, as it must be used in conjunction with more effective mosquito control methods to repel mosquitoes. |
| Mosquito repellent [44],Bug Zappersan electrical discharge insect control system [45], [46].The bug zapper actually kills harmless and beneficial bugs. This makes this device an ineffective form of mosquito control [47]. Scientific studies prove that mosquitoes are attracted to carbon dioxide, heat and moisture, not to sound or light [48]. Mosquito nets [49], [50], [51] an effective barrierwhen the entrance and exit areas are not breached by human traffic. Moreover, the coverage is limited to the area that the net covers and Mosquito Magnet® trap [52]a Mosquito Magnet trap significantly decreases the mosquito population by killing enough mosquitoes to interrupt their breeding cycle. This mosquito trap is made to mimic a warm-blooded creature by giving off carbon dioxide and a sweat scent as well as heat. These devices are still in the research and development phase. |
| As illustrated from the epistle of temporary control measures, success can be as low as 2%, the mosquito control methods may only be effective at repelling or preventing bites, not actually killing the mosquitoes; furthermore, the protected area is small and there is a limit to the amount of protection time. |
| Therefore, there is great urgency for the development and implementation of mosquito vector control interventions until a satisfactory solution is attain.[53] reports the importance of mathematical models as a first step to asses control strategies and the efficacy of proposed methods prior to implementation.[54]proposed a system of ordinary differential equations to model the interactions among the larval and adult stages of mosquitoes and water mites and [55]have developed mathematical models to show that it is a crucial element in developing optimised control techniques, especially to understand the pest population dynamics, which controls the strength of the disease vector. |
| Natural control methods |
| Due to the ineffectiveness of the control measures that we have reviewed, it is imperative to develop a Natural Control Model (NCM), which is based on the deployment of mosquito predators. Odonata and Toxorhynchitesare both naturally beneficial predators of all classes of mosquitoes [56]; hence, we investigate their effectiveness in the following model. |
| The NCM concept provides a general opportunity for the control of all species of mosquitoes as it is based on the interaction between the population of all species of mosquito adults and its life cycle stages (egg, larvae and pupae) and the naturally beneficial predator Odonataadult and its life cycle stages (egg and nymph) and Toxorhynchites adult and its life cycle stages. Fig. 1 illustrates the predatory action of Odonata on the mosquitoes and Toxorhynchitesand the predatory action of Toxorhynchiteson the disease vector mosquito larva. The green arrows indicate the predation of Odonata nymphs on pest mosquito and Toxorhynchites: eggs, larvae and pupae. The orange arrows indicate the predation of Odonata adults on disease vector mosquitoes and Toxorhynchites adults. The red arrow indicates predation of Toxorhynchiteson disease vector mosquito larvae. |
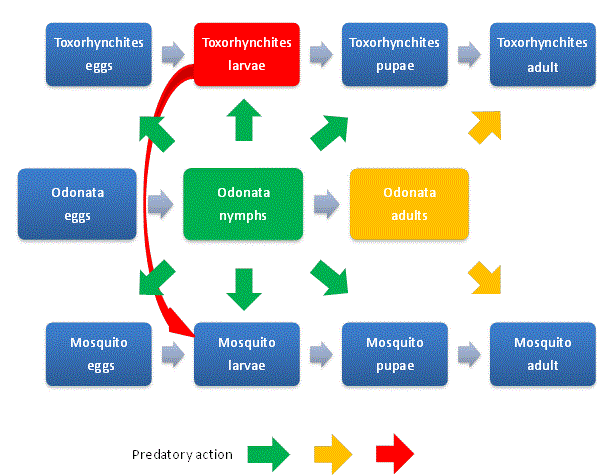 |
MATERIALS AND METHODS |
| To understand the mosquito challenge, time was spent assessing the problems in Atimbo a residential area, located in Calabar; the capital city of Cross River State, Southern Nigeria. A more than 100 km2 residential area is filled with ideal breedingplaces for mosquitoes, open grassy areas are found to feature unmanaged gardens and tree waste, discarded food waste, recyclable containers, flower pots, birdbaths, water dishes, cans, gutters, tires, buckets, marsh areas and puddles in the drainage areas, as illustrated in Fig. 2 |
 |
| Because the population of insects and mosquitoes observed in the Atimbo area were so numerous, we collected some species samples and presented them to the pest detection system, developed by [57]. The results obtained from the detection system confirmed the presence of more than one species of disease vector mosquito, see Fig. 3. |
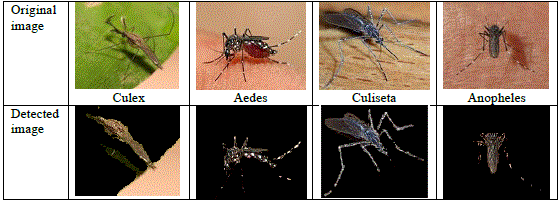 |
| Model of the interaction between the mosquitoes and predators |
| The Odonata adult and nymph are recommendedbecause the Odonata nymph is very effective in reducing the mosquito population by eating the mosquito: eggs, larva and pupa, see Fig. 1. Odonata adults are very important predators and a valuable ally for humanity as they feed on adult mosquitoes, especially when their populations are in abundance. [58] experimentally confirmed the feeding ability of the Odonata nymph for the control of several species of mosquitoes. [59] discussed the agility of the dragonfly compared to mosquitoes and houseflies as thus: “the dragonfly is many times the size of a mosquito or a housefly and only needs to flap its wings a mere 30 times a minute when compared to a mosquitoâÃâ¬ÃŸs 600 times a minute and the houseflyâÃâ¬ÃŸs 1000 flaps a minute to maintain flight with peak manoeuvrability. Such is its power that the dragonfly is equipped with a low-energy, high speed capability; very few insects can escape its basket shaped grabbing limbs that it uses to clutch on to its prey before crushing them into a gooey mass, with its powerful mandibles and swallowing it”. |
| Toxorhynchites are unique in that none feed on blood and, unlike many other mosquitoes, they are harmless to mankind. The larvae of all Toxorhynchites are predaceous on other mosquito larvae or small aquatic arthropods, they are therefore beneficial to humankind, see Fig. 1. They have the ability to consume 10 to 20 mosquito larva in a day and up to 5000 during their whole larva stage [60], [61], [62]. |
| Our goal is population reduction as recommended by [63], [64]. Therefore the following equations provide a dynamic model of the evolving disease vector mosquito life stages, Odonata and its life cycle stages and Toxorhynchites adult and its life cycle stages per square kilometre. The model includes reproduction, mortality and predation using Weibull probability distribution function and Pascal (negative binomial) distributions. |
| The Pascal (negative binomial) distribution evaluates the distribution of the number of trials needed to get a fixed number of successes (r) [65], [66]. The negative binomial distribution has been found to be useful in several areas of study, including the fitting of several frequency distributions of different biological data [67]. The enquiry into the frequency distribution of multiple events with particular reference to the occurrence of multiple attacks of disease or repeated accidents[68]. The consideration of the problem of mortality curve fitting over the entire life range [69], [70]analysed organism frequency count data using the negative binomial distribution. |
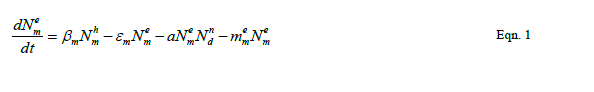 |
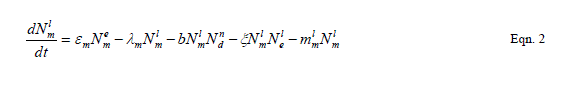 |
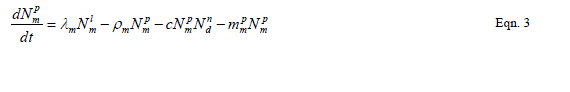 |
| The proposed model consists of eleven simultaneous non-linear, ordinary differential equations (1) to (11), which are solved using a 4th order Runge–Kutta method as described by [71], [72], [73]. Using the average life span of all the insect life cycle stages and their mortality rates as described in the previous works of [74]. The following results were obtained from the combination of tables 1, 2 and 3, and the Weibull probability distribution function to determine the various mortality rates of the pests and predators; for the detailed procedure refer to [75]. |
 |
FREQUENCY OF CAPTURE |
| To determine the frequency with which predators capture prey, we used a negative binomial distributionfunction (pdf),equation 12 and the negative binomial cumulative distribution function (cdf) equation 13, and the mean equation 14. |
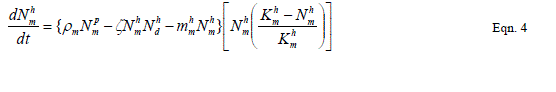 |
| Where eqn. 12 and eqn.13 returns the negative binomial pdf and cdf at each of the values of „x’ using the corresponding number of successes, „r’ and probability of success in a single trial, „pâÃâ¬ÃŸ. Where X is the number of trials needed to achieve a particular success rate „râÃâ¬ÃŸ |
| This models the scenario for the successive random trials that the predator undertakes, with each predation attempt having a probability of success „pâÃâ¬ÃŸ. The number of attempts that the predator must perform in order to capture a given number of prey r has a negative binomial distribution where „IâÃâ¬ÃŸ is the indicator function, which ensures that „râÃâ¬ÃŸ only adopts integer values. |
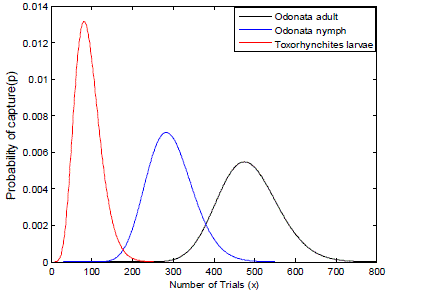
| Therefore, considering the life span of the predators Toxorhynchites larvae, and Odonata adult and nymphs and their feeding habits extracted from the research works as shown above. The frequency of capturing their prey can be modelled using the negative binomial function as illustrated in Fig. 4. |
 |
EXPERIMENTAL RESULTS |
| Scenario I |
| With no initial infestation, the area is invaded by over two million adult mosquitoes, each laying an average of 175 eggs per day. The results obtained in Fig. 5 illustrate the reproductive capability of a typical pest mosquito, which has had its blood meal. Since there were no control measures in place to manage the mosquito growth rate, within the space of 20 days the mosquito population density reaches its carrying capacity of 4.0 *107. |
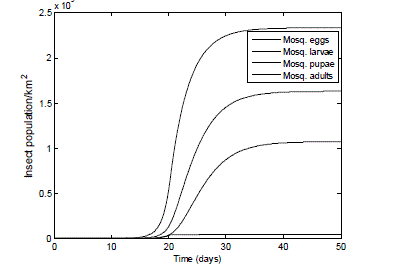 |
| Fig. 5 Population of adult mosquitoes in favourable conditions, starting with zero population of: eggs, larva & pupa . The graph shows the reproduction rate of a typical female mosquito pest after obtaining a blood meal. The adult laid its eggs which transform into larvae then to pupae and finally into adults. |
| Scenario II. |
| This illustrates how an already established infestation level develops, as would be the case in the conditions around the residential areas of the Atimbo community, illustrated by Fig. II. We estimate the mosquito (adult, eggs, larvae and pupae) population densities to be at least above 2,000,000 females per km2, with each female mosquito laying an average of 175 eggs per day. In the absence of any control measure, the simulated results are shown in Fig. 6. |
| The result of Fig. 6 shows a great increase in the population of the mosquito: eggs, larvae, pupae - from 2,200,000 to 2.33*109 eggs; 1.63*109 larvae, 1.07*109 pupae and the adult population reaches the carrying capacity of 4.0 *107 in the environment within 6 days. It is hardly surprising that the health of people living in this dwelling area is very poor. |
| Scenario III. |
| The model was set up with different populations of Odonata and Toxorhynchite. The results reported in Fig.7 are for a starting population of 200 Odonata adult and its life cycle stages and 100 adult Toxorhynchitesand its life cycle stages. |
| In Fig. 7 with the introduction of 200 Odonata adult and all its life cycle forms and 100 Toxorhynchites adults and its life cycle stages the mosquito population density was controlled; the population was observed to drop from a peak of 1.35*109 to 560 eggs, 8.14*108 to 372 larvae, 4.87*108 to 207 pupae and 2.90*107 to 4 adults in 90 days. |
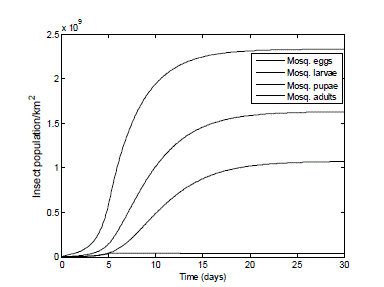 |
| Fig. 6 Mosquito population in the absence of any control measures or predators. The graph of Fig.6 demonstrates the reproduction rate of the female mosquitoes in an established infestation where there is an initial starting population of all the life cycle stages. Because of the initial starting population, the adult pest reaches the carrying capacity earlier when compared to Fig. 5. |
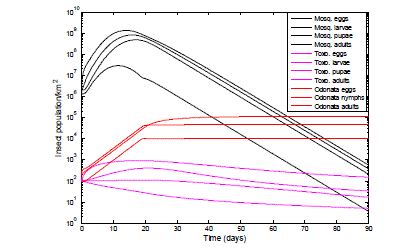 |
| Fig. 7 Effect of the introduction of Toxorhynchites: adult, eggs, larvae & pupae and Odonata: adult, eggs & nymph on mosquitoes infestation. The graph of Fig. 7 shows thesemilog plot of the control efficiency of the combined predators when introduced into the mosquito infected habitat. From the legends the black colour lines with the title Mosq., represents the mosquito population of the adults and its life cycle stages in the presence of the predator Odonata shown with the red coloured lines and the predator Toxorhynchites (Toxo)., shown withmagentacoloured lines. |
| There is predation of Toxorhynchites adults and its life cycle stages by Odonata, which is evident from the results of Fig.7, this is incorporated into equations (5) to (8). 100Toxorhynchites adult and life cycle stages (egg, larvae and pupae) were introduced into the mosquitoes infested habitat, the maximum population observed for the Toxorhynchites eggs, larvae and pupae were 905 eggs, 404 larvae and 109 pupae for the first 30 days. Out of the total population of 100 Toxorhynchites deployed, the population at 90 days is 148 eggs, 33 larvae, 17 pupae and 5 adults. These findings are consistent with the experimental work of [83], [84], [85], which reports that predation occurs between predators. |
| The results of Fig. 5 and Fig. 6 show that mosquito have the ability to multiple uncontrolably expecially if a blood meal is obtained as discussed earlier. When any pest mosquito specie is sighted action should be taken. |
| The result of Fig. 7, illustrate a successful reduction of the population of mosquitoes as the predators established their population in the environment. The result demonstrates the possibility of obtaining a healthier environment with the deployment of Odonata and Toxorhynchites species into mosquito infested environments. |
| The combination of predators controls the mosquito population and demontrates the great potential of this strategy to significantly reduce the mosquito life cycle stages in a reasonable number of days. This duplex approach requires fewer Odonator preditors to be deployed, reducing the resources required to achieve an impressive result. This proposal considers both long and short term permanet approach to resolve the problems of mosquitoes. Further research in this area will consider an immediate solution along side the long term permanent solutions. |
CONCLUSION |
| It is observed that as long as an environment is left uncared for, it will definitely become a breeding ground providing many mosquito hatcheries. When an environment is occupied with millions of mosquitoes, no preventive measures can cure or manage the mosquito pest. Therefore it is recommended that in addition to managing the environment and preventing it from becoming a breeding zone, a permanent control measure with a combination of predators should be employed. |
| Breeding, then deploying beneficial insect predators like the Odonata andToxorhynchites will transform a hostile environment into a zone habitable by humans, as these predators have a great ability to control mosquitoes at low cost and in a short amount of time. We also advise the restoration of natural habitats that will attract the visits of important naturally beneficial insects. |
| Finally patient must be exercised to breed beneficial insects as it takes several days before significant results will be noticed and to achieve a mosquito free environment. Any form of insecticides must be avoided in order to preserve the life span of the predators. The goal should be a world free of: disease vector mosquito species and chemical pesticides. |
References |
|