ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Haizel G. Roy Higher Secondary School Teacher, Department of Chemistry, Government Model Higher Secondary School, Muvattupuzha-686661, Ernakulam, Kerala, India. |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
TiO2 nanostructures with flower like morphology was synthesized by a simple microwave assisted acid hydrolysis of TiCl3. Tuning the morphology was achieved by the microwave treatment and the nature of the medium or the precipitating agent. As-synthesized titania nanoflowers, was characterized by X-ray diffraction (XRD), UV-Visible spectroscopy, Infrared spectroscopy (IR) and Scanning Electron Microscopy (SEM). The BET surface area, pore size distribution and pore volume of the samples were measured using a static volumetric system, Micromeritics ASAP 2010 equipment. The as-prepared TiO2 nanoflowers appear to be single crystalline phase and the diameter is about 33.55 nm. The Photocatalytic activity studies reveal that the as-synthesized rutile titania nanoflowers show higher photocatalytic activity. In most cases, rutile TiO2 nanoparticles show poor photocatalytic activities than the pure anatase phase. Interestingly, the single phase rutile TiO2 nanocrystals with flower like morphology formed in the absence of any additives exhibited higher photocatalytic activity than the famous commercial photocatalyst Degussa P- 25 on the photocatalytic degradation of Methyl Red.
Keywords |
| Microwave Irradiation, Methyl Red, Nanoflower, Nanotitania, Optical Property, Photocatalytic activity |
INTRODUCTION |
| Titanium dioxide (TiO2) is widely used as an important material. It is well known that titanium dioxide exists in three main polymorphic phases; rutile, anatase and brookite. Rutile and anatase are the most commonly synthesized phases. Anatase and brookite are metastable phases and convert it into rutile at high temperature, usually above 600οC [1]. Rutile is a thermodynamically stable phase with a smaller band gap (3.0 eV), used as the main white pigment in paints and cosmetic products because of the basic inert, non toxic and high light scattering and refractive index [2]. |
| A large number of methods have been developed for the synthesis of TiO2 nanoparticles, for example, sol-gel process [3], hydrothermal methods [4, 5], solvothermal methods [6] and emulsion precipitation [7]. Although TiO2 nanoparticles have been prepared successfully via these methods, most of the nanoparticles synthesized via traditional routes are poorly crystalline and/or exhibit a broad size distribution. For obtaining single phase rutile, Li et al [8] used rutile seed crystals to crystallize nanocrystalline rutile below 95οC. Synthesis of rutile below 65οC after treatment for 6 h was reported by Kim et al. [9]. Wang et al. [10] used mixtures of hydrochloric acid and alcohol aqueous solutions to obtain rutile in the temperature range of 40-90 οC. Pottier et al. [11] used concentrated HCl solutions of TiCl4 to synthesize nanomeric particles of rutile and brookite. Thus the crystallization of rutile in acidic media involved seed crystals, long times, alcohol, concentrated HCl solutions etc. Sometimes, mineralizers such as SnO2 [12], NH4Cl or NaCl [13] has been added to reduce particle size. But this could cause contamination of titanium dioxide crystals. |
| In recent years, TiO2 has received much attention as a photocatalyst. The photocatalytic activity of TiO2 depends on its crystal structure, and hence several studies have been carried out on the dependency [14-19]. More recently, it has been found that a mixture of anatase and rutile TiO2 nanoparticles has a much higher photocatalytic activity than pure anatase or pure rutile TiO2 nanoparticles. In most cases, rutile TiO2 showed poor photocatalytic activities than the anatase phase [20, 21]. |
| In the present work, I report a simple microwave irradiation method to synthesize phase pure rutile nanotitania from 1 M HCl in the absence of any additives to obtain nanotitania with small crystallite size and an attractive flower like morphology. The optical properties and photocatalytic activity studies were also discussed in detail in the present study |
II. EXPERIMENTAL |
| A. SYNTHESIS |
| All reagents were purchased from Merck, Germany. Titanium trichloride (15 wt% TiCl3, 10 wt% HCl) were used as, the titanium precursor and IM HCl solution was used as the precipitating agent. A typical microwave oven (Whirlpool, 1200W) operating at a frequency of 2,450 MHz was used for the synthesis. 10 mL TiCl3 was added drop wise to 250mL of 1M HCl solution and it was irradiated in a microwave oven in on and off mode for 15 minutes for complete precipitation. The completion of the reaction is checked by noting the colour change (blue to colourless) of the reaction mixture. The white precipitate formed was aged for 24h and washed thoroughly with distilled water. The precipitated titania was then dried in an air oven at 100οC and further calcined in a muffle furnace at 300οC for 4 hours. |
| B. CHARACTERIZATION |
| The X-ray diffraction (XRD) patterns of the titania were recorded on a Bruker D8 advanced diffractometer with Cu Kα radiation. The crystallite size of TiO2 was calculated using Debye Scherrer equation, L = κλ/βcos θ, where L is the average crystallite size, λ is the wavelength of the radiation, θ is the Bragg’s angle of diffraction, β is the full width at half maximum intensity of the peak and κ is a constant usually applied as ~0.89. Scanning electron microscopic images were taken in a JEOL JSM-5600 SEM equipped with energy dispersive X-ray analysis (EDX). IR spectra were recorded using Schimadzu 8400 FTIR spectrophotometer in the range of 400-4000 cm-1. The ultraviolet-visible absorption (uv-vis) spectra were recorded using a UV-2450 Schimadzu UV-Visible spectrophotometer. TG and DTA measurements were carried out using shimadzu DTG-60 equipment. DSC measurements were carried out using Schimadzu DSC-60 equipment. The BET surface area, pore size distribution and pore volume of the samples were measured using a static volumetric system, Micromeritics ASAP 2010 equipment. For the characterization, nitrogen adsorption isotherm at 77 K in the pressure ranging from 0.1 to 760 mm Hg was measured. The total pore volume was calculated from the amount of nitrogen adsorbed at relative pressure of 0.97. The total surface area was calculated using multi point Brunauer Emmett Teller (BET) surface area method. The pore size distribution was calculated using Barrett Joyner Halenda method. |
| C. PHOTOCATALYTIC ACTIVITY STUDIES |
| 250 mg of TiO2 is added to 250 ml of an aqueous solution of 2 x 10-6 M Methyl Red (MR) solution with vigorous stirring. To maximize the adsorption of the dye on to the TiO2 surface, the resulting mixture was kept in the dark for 30 minutes. The methyl red solution was then collected by centrifugation and C0 was measured by UV-visible spectroscopy. The methyl red solution including TiO2 was stirred at intervals. To plot Ct/C0 against illumination time, the suspension was collected by centrifugation and absorption was measured at 15 minutes time intervals for 3 hours. |
III. RESULTS AND DISCUSSION |
| X-RAY DIFFRACTION |
| Figure 1 shows the X-ray diffraction (XRD) pattern of the synthesized titania nanoflowers synthesized by microwave irradiation method. The X-ray diffraction (XRD) patterns of the TiO2 nanoparticles show that rutile structure is formed when 1 M HCl is used as solvent. The average crystallite size of the rutile titania is calculated from X-ray line broadening by the Debye Scherrer equation, on the (110) diffraction peaks [22, 23]. The average crystallite size is found to be 33.55 nm. All the peaks can be readily indexed to the pure rutile phase with lattice constants a = 4.548 Å and c = 2.946 Å, which are basically in agreement with the reported values (JCPDS no.88-1173). The absence of any other peak indicates the phase purity of the synthesized nanotitania. |
 |
| BET SURFACE AREA ANALYSIS |
| Figure 2 shows the N2 sorption isotherms of the synthesized titania nanostructures with their corresponding pore size distribution curves calculated from the desorption of the N2 sorption isotherms by the BJH (Barrett-Joyner-Halenda) method. The BET surface area reveals that rutile nanoflowers synthesized from 1M HCl has a pore size of 6.2 nm . The BET surface area and pore volume of the synthesized nanotitania are 13.37m2g-1 and 0.37 cm3g-1 respectively. |
 |
| SCANNING ELECTRON MICROSCOPY |
| Figure 3 shows a typical SEM image of the as prepared titania nanoflowers. The SEM image clearly indicates the flower like morphology of the synthesized nanotitania. The SEM image indicates that the nanoflowers are quite clean with no contamination attached to their surface |
 |
| FTIR SPECTRA |
| The FTIR spectra of rutile titania synthesized from 1M HCl is are shown in figure 4. The FTIR spectrum Show a broad band around 3398 cm-1, which is attributed to the O-H stretching mode of the surface, adsorbed water molecules. This is supported by another band at 1631 cm-1, which is attributed to the O-H bending mode. The band at 428 cm-1 is due to the Ti-O bond stretching mode of rutile titania. |
 |
| OPTICAL PROPERTIES AND BAND GAP STUDIES |
| DIFFUSE REFLECTANCE SPECTRA |
| The diffuse reflectance spectra of the synthesized titania nanostructures are shown in figure 5. In this spectrum absorption maximum is observed at 293 nm. From these spectra it is clear that a blue shift is observed with decreasing particle size of nanotitania [24]. |
 |
| QUANTUM SIZE EFFECT AND BAND GAP STUDIES |
| For a semiconductor, the electron excitation consists of a loosely bonded electron-hole pair, usually delocalized over a length much longer than the lattice constant. As the diameter of the semiconductor crystallite approaches this exciton Bohr diameter, its electronic properties start to change. This is so called quantum size effect, which can be observed as a blue shift in the optical band gap. |
| The band gap (Eg) of a semiconductor can be estimated from the plot of (ïÃÂáhïÃÂî)2 versus photon energy (hïÃÂî). The band gap energy is determined by extrapolating the curve to the x-axis, as shown in the figure 6. The absorption spectrum of rutile nanotitania shows a lower absorption and the calculated band gap is around 3.15 eV, which is slightly higher than the reported value for rutile titania. The higher band gap could be a result of small particle size and the flower like morphology. |
| THERMAL STUDIES |
| The combined TG-DTA and DSC curves a shown in figure 7. The TG curve shows a mass loss of 13.36% that may be due to the loss of adsorbed water. The DTA curve shows one endothermic peak at the beginning, which can be attributed to the loss of water molecule present in the sample. From the DTA curve it is clear that the sample has no phase change even at high temperature. The same changes are observed in the DSC curves. |
 |
| PHOTOCATALYTIC ACTIVITY STUDIES |
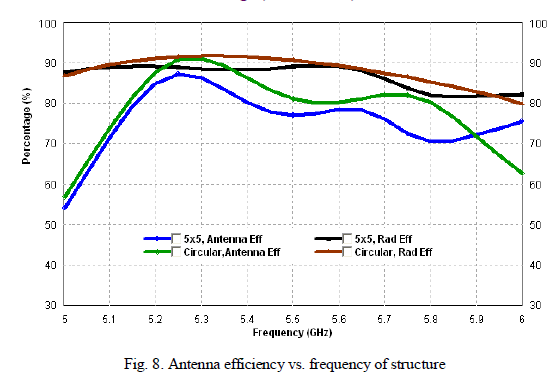
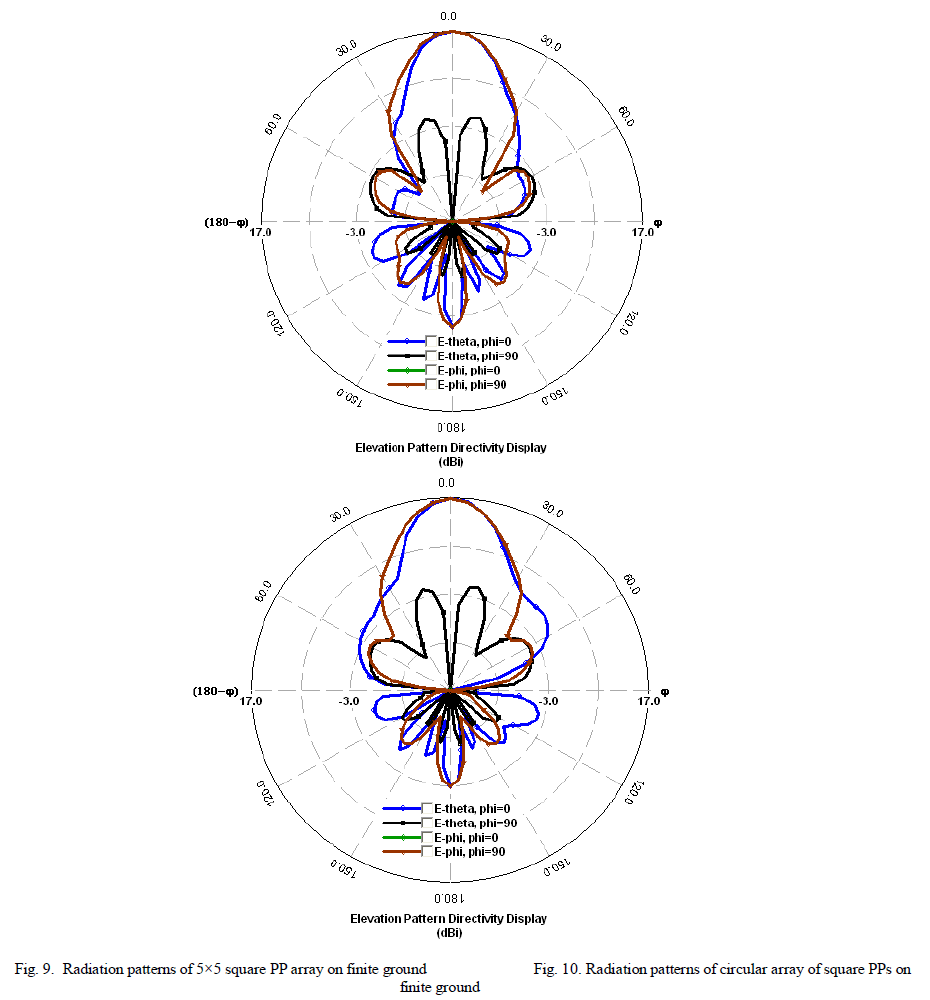
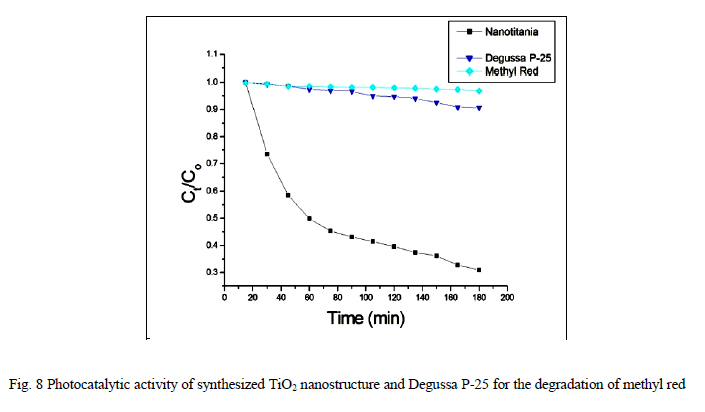
| Photocatalytic processes involve irradiation of a semiconductor such as TiO2 with energy greater than or equal to the band gap of the semiconductor. This promotes electrons from the valence band to the conduction band, generating photoexcited electrons (e−) and holes (h+). The photoexcited electrons and holes may diffuse to the surface of the semiconductor, followed by interfacial electron transfer to and from adsorbed acceptor and donor molecules. The holes are involved in the oxidation reactions, typically the mineralization of organic substances present in the solution [25]. In the present work photocatalytic activity tests were conducted by the self-sensitized degradation of the dye, Methyl Red in aqueous solution under ultraviolet light irradiation. Methyl Red (MR) shows a maximum absorption at 437 nm. The absorption peak gradually diminishes upon the ultraviolet light irradiation, illustrating the degradation of Methyl Red. The concentrations of methyl red with irradiation time for the rutile titania nanostructure, Degussa P-25 and methyl red are shown in Fig.8 It is reported that among the three crystalline phases of TiO2, the anatase phase has higher photocatalytic activity [26]. In most cases, rutile TiO2 nanoparticles show poor photocatalytic activities than the pure anatase phase. Interestingly, the as-synthesized single phase rutile TiO2 nanocrystals with flower like morphology formed in the absence of any additives exhibited higher photocatalytic activity than the famous commercial photocatalyst Degussa P-25 on the photocatalytic degradation of Methyl Red. The photocatalytic activity of the synthesized sample is related to their surface area and particle size. Small particle size not only produces high surface area but also shortens the route on which an electron from the conduction band of TiO2 migrates to its surface. Moreover, high surface area of TiO2 can provide more active sites and absorb more reactive species. |
 |
IV. CONCLUSION |
| A simple and efficient microwave method has been developed for the synthesis of nanotitania with a flower like morphology. Nanotitania with a flower like morphology, small particle size and surface area can be effectively synthesized by microwave irradiation technique. The synthesized nanotitania was structurally and physicochemically characterized. XRD analysis reveal that the titania nanostructure have average crystallite size of 33.55 nm. The porosity of the synthesized titania nanostructure were determined by BET and BJH method. The SEM image clearly reveals that the sample has a flower shaped morphology. The photocatalytic activity was studied by the self-sensitized degradation of methyl red (MR) in aqueous solution under UV light irradiation. The synthesized rutile TiO2 with high surface area exhibit higher photocatalytic activities than Degussa P-25 photocatalyst. |
ACKNOWLEDGMENT |
| The author is grateful to the School of Chemical Sciences, Mahatma Gandhi University Kottayam, for the XRD, IR, UV-Vis and Photocatalytic studies and Regional Research Laboratory, Trivandrum for SEM imaging. The author is also grateful to The State Central Library & University Library, Thiruvananthapuram, Kerala, India for providing books and journals to carry out this research work. |
References |
|