e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
1Dr. B.C. Roy College of Pharmacy & Allied Health Sciences (BCRCPAHS), Durgapur - 713206, West Bengal, India.
2Chemical Technology Dept., Calcutta University, Kolkata-700 009, West Bengal, India.
3Fresenius Kabi Oncology Ltd., Kalyani,-741235, West Bengal, India.
Received: 14/06/2013; Revised: 26/06/2013; Accepted: 04/07/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Skin is an important site of drug application for both local and systemic effects. The idea of transdermal drug delivery system (delivering drugs through skin) is old, as the use of it is reported back in16th century B.C. The first transdermal patch was approved by FDA in 1979. It contained the drug scopolamine, used to treat motion sickness. The success of a dermatological drug to be used for systemic drug delivery depends on the ability of the drug to penetrate through skin in sufficient quantities to achieve the desired therapeutic effect. Ondansetron Hydrochloride is a 5-HT3 antagonist (5-HT3-receptor antagonist) with antiemetic activity. It is used in the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy. The matrix-type transdermal patches containing Ondansetron HCl were attempted to prepare using different ratios of Ethyl cellulose, Hydroxy propyl methyl cellulose (E15) and plasticizer. Glycerol was used as plasticizers. Five such formulations of Ondansetron HCl transdermal patches were formulated using different polymeric ratios. The formulations were subjected to evaluation for physico-chemical parameters like thickness, weight variation, drug content, folding endurance, % moisture content, moisture uptake, flatness water vapor transmission rate, and biopharmaceutical evaluation like in-vitro drug release study, in-vitro permeation study through dialysis membrane. Matrix patches showed an initial burst effect to provide the loading dose of the drug, followed by sustained release, indicating a promising potential of the Ondansetron hydrochloride matrix patches as an alternative to the conventional dosage form like tablets.
ondansetron, optimization, transdermal
Skin is an important site of drug application for both local and systemic effects. The idea of transdermal drug delivery system (delivering drugs through skin) is old, as the use of it is reported back in16th century B.C [1]. During the last years, developments in transdermal drug delivery have been incremented focusing mainly on overcoming problems associated with the skin barrier properties [2,3]. In skin, the stratum corneum is the main barrier for drug penetration. The success of a dermatological drug to be used for systemic drug delivery depends on the ability of the drug to penetrate through skin in sufficient quantities to achieve the desired therapeutic effect.
One patch design consists of four layers of thin, flexible membranes: an impermeable backing, a drug reservoir, a rate-controlling membrane, and an adhesive. When the patch is applied, the drug begins flowing through the skin into the bloodstream at a rate regulated by the membrane, pre-programmed to keep the drug at levels that provide effectiveness with acceptable adverse effects.
Transdermal drug delivery systems offer several important advantages over more traditional approaches, including:
• Longer duration of action resulting in a reduction in dosing frequency.
• Increased convenience to administer drugs which would otherwise require frequent dosing.
• Improved bioavailability.
• More uniform plasma levels.
• Reduced side effects and improved therapy due to maintenance of plasma levels up to the end of the dosing interval.
• Flexibility of terminating the drug administration by simply removing the patch from the skin.
• Improved patient compliance and comfort via non-invasive, painless and simple application.
• Prevent the first-pass metabolism of drug.
• When drug is impossible to take orally like in continuous vomiting condition and unconscious patient.
• Possibility that a local irritation at the site of application
• Erythema, itching, and local edema can be caused by the drug, the adhesive, or other excipients in the patch formulation.
• Increase in transepidermal water loss (TEWL).
Ondansetron Hydrochloride is a 5-HT3 antagonist (5-HT3-receptor antagonist) with antiemetic activity. It is used in the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy. Ondansetron Hydrochloride is also used for the prevention and treatment of postoperative nausea and vomiting. Furthermore, Ondansetron Hydrochloride is used for the management of nausea and vomiting, and the important role of 5-HT3 antagonists. As long term medication is required to prevent the nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy. So transdermal matrix patch of Ondansetron HCl may be alternative of oral drug delivery system of antiemetic drug.
The objective of the present study deals with the formulation and evaluation of Ondansetron HCl transdermal matrix patches using ethyl cellulose (EC), hydroxyl propyl methylcellulose (HPMC E15) by solvent evaporation technique by keeping the concentration of the drug constant.
Ondansetron hydrochloride was received as gift sample from N.I.Laboratories, Kolkata. Ethyl cellulose, Hydroxy propylmethyl cellulose (HPMC-E15), Glycerol were recieced from Loba Chemie Pvt Ltd., Mumbai. All other chemicals and solvents used were of analytical grade.
Drug-excipient compatibility study was conducted with FTIR spectrum.
To prepare the backing membrane 3% polyvinyl alcohol was dissolved in warm distil water. Then 2ml of this solution was transferred in each glass mold after filtering the solution. There after it was dried in a drier at 60°C for 6 hr.
The matrix-type transdermal patches containing Ondansetron HCl were prepared using different ratios of ethyl cellulose, Hydroxy Propyl methyl cellulose E15 and plasticizer. The polymers in different ratios were dissolved in the solvent (chloroform). Glycerol was used as plasticizers. Then the drug was added slowly in the polymeric solution and stirred on the magnetic stirrer to obtain a uniform solution. Then the solution was poured on the glass mold having backing membrane and dried at the room temperature. Then the patches were cut out from glass mold for evaluation [3].
10mg of Ondansetron Hydrochloride was accurately weighed and was dissolved in 10ml of methanol. The solution was then diluted using phosphate buffer (pH-7.4) to 100ml. The solution was scanned then in the wavelength range of 200-600nm in UV-VIS 1800 Spectrophotometer, Shimadzu, Japan.
The thickness of transdermal film is determined by dial calipers at different points of the patch.
Weight variation is determined by individually weighing 6 randomly selected patches and calculating the average weight and standard deviation. The individual weight should not deviate significantly from the average weight [5].
An accurately weighed portion of patch (about 100 mg) is dissolved in 100 ml of phosphate buffer and then the solution is shaken continuously for 24 h on shaker. Then the whole solution is sonicated. After sonication and subsequent filtration, drug in solution is estimated spectrophotometrically by appropriate dilution [6].
10 patches are selected and content is determined for individual patches. Thereafter, it is observed i.e., 9 out of 10 patches have content of drug between 85% to 115% of the specified value and one has content beyond 75%-125% of the specified value, then transdermal patches pass the test of content uniformity. But if 3 patches have content in the range of 75% to 125%, then additional 20 patches are tested for drug content. If these 20 patches have range from 85% to 115%, then the transdermal patches pass the test.
The prepared patches are weighed individually and kept in a desiccators containing silica gel at room temperature for 24 h. The films are weighed again after a specified interval until they show a constant weight. The percent moisture content is calculated using following formula [7].

Weighed patches are kept in a desiccator at room temperature for 24 h. These are then taken out and exposed to 84% relative humidity using saturated solution of Potassium chloride in a desiccator until a constant weight is achieved. % moisture uptake is calculated as given below [7].
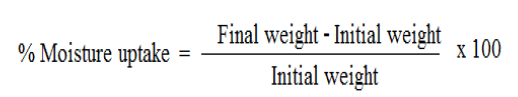
A transdermal patch should possess a smooth surface and should not rict with time. This can be demonstrated with flatness study. For flatness determination, one strip is cut from the centre and two from each side of patches. The length of each strip is measured and variation in length is measured by determining percent constriction. Zero percent constriction is equivalent to 100 percent flatness.
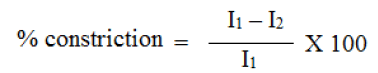
I2 = Final length of each strip
I1 = Initial length of each strip
Evaluation of folding endurance involves determining the folding capacity of the patches subjected to frequent extreme conditions of folding. Folding endurance is determined by repeatedly folding the patches at the same place until it break. The number of times the patches could be folded at the same place without breaking is recorded as folding endurance value [8].
• Conical flasks of equal diameter were used as transmission cells.
• About 1gm of anhydrous calcium chloride was placed in each flask.
• The prepared transdermal patches of each formulation were fixed over the brim.
• The cells were accurately weighed and kept in 84% RH.
• The cells were taken out and weighed after 6, 12, 24, 36, 48 and 72 hrs of storage.
• Water vapor transmission rate is calculated as the number of grams of moisture gained/h/cm>sup>2.
The sections of each sample are cut and then mounted onto stubs using double sided adhesive tape. The sections are then coated with gold palladium alloy using fine coat ion sputter to render them electrically conductive. Then the sections are examined under scanning electron microscope.
The in-vitro release study was done by the Keshary Chien diffusion Cell method. In this method transdermal system is placed in between receptor and donor compartment of the diffusion cell. The transdermal system faces the receptor compartment in which receptor fluid i.e., buffer is placed. The whole assembly is kept on magnetic stirrer and solution in the receiver compartment is constantly and continuously stirred with 600 rpm throughout the experiment using magnetic beads. The temperature of receptor compartment is maintained 37 ± 2°C. At predetermined time intervals, the 5ml receptor fluid is removed for analysis and is replaced with an equal volume of phosphate buffer. The concentration of drug is determined spectrophotometrically at 249 nm wavelength with suitable dilution [10,11].
In-vitro permeation studies were carried out using modified Keshary Chien diffusion cell. The dialysis membrane was previously soaked for 24 hours in distilled water. The patches were adhered to the barrier membrane (dialysis membrane) and the membrane is tied firmly to the donor compartment of the Keshary Chien diffusion cell, the receptor compartment of which is filled with 57 ml phosphate buffer. The donor compartment is lowered to the receptor compartment in such a way that the dialysis membrane just touches the media of the receptor compartment. The total setup was placed on a magnetic stirrer. The temperature of receptor compartment is maintained 37 ± 2°C. The content of the diffusion cell was stirred using a teflon coated bead at a constant speed (600 rpm). Samples were withdrawn (5 ml) at predetermined time intervals and replaced with same amount of phosphate buffer to maintain the sink condition. After suitable dilution, the samples were analyzed for drug content using UV-VIS spectrophotometer at wavelength 249 nm. The permeation study was carried out for 8 hours [12].
Five formulations of Ondansetron HCl transdermal patches were formulated using different polymeric ratios. The formulations were subjected to evaluation for physico-chemical parameters like thickness, weight variation, drug content, folding endurance, % moisture content, moisture uptake, flatness water vapor transmission rate, and biopharmaceutical evaluation like in-vitro drug release study, in-vitro permeation study through dialysis membrane.
The thickness of the patch was determined by dial calipers at different points of the patch. The thickness of the patches varied from 0.44 to 0.56 mm. The values obtained for all the formulations are given in table 2. As there was decreasing in HPMCE15 and increasing in EC, there is consistent increase in thickness. So, it is evident from the data that EC help in increasing in the thickness of the patch.
The weight variation was to be in range of 350.67 ±10.12 mg to 406.28±11.67 mg. The values for all the formulations are tabulated in table 2. As there was increasing EC, there is consistent increase in weight because of low water permeability nature of EC that prevent evaporation of water.
Folding endurance of all the formulation were found to be satisfactory and it was in the range of 8.83±1.47to 24±1.05. The values for all five formulations are given in the table.The folding endurance value of all the patches was found satisfactory which ensures that patches prepared using plasticizer glycerol (40 % w/w of polymer)were having optimum flexibility and were not brittle.
The moisture content is increased as hydrophilic polymer concentration increased and similarly decreased as hydrophobic concentration increased. Among all the patches OH 1 was found to highest moisture content (12.01±.14). The formulation OH 5 (EC: HPMC E15 2:1) showed lowest percent moisture content than other formulations. This might be because of the low water permeability of ethyl cellulose polymer. Lower moisture content in formulations is reasonably good for a transdermal patch to prevent brittleness with 100% dryness, maintain stability. Greater amount moisture content can lead to microbial contamination during storage.
The moisture uptakes of Ondansetron HCl patches as a function of HPMC E15 /EC ratios are presented in table 2. The moisture uptake is increased as hydrophilic polymer concentration increased and similarly decreased as hydrophobic polymer concentration increased. Among all the patches OH 1 was found to highest moisture content (9.82±.43). The formulation OH5 (EC: HPMC E15 2:1) showed lowest percent moisture content than other formulations. This might be because of high affinity of HPMCE15 for water.
The formulation OH 5 (EC: HPMCE15 2:1) showed maximum water vapor transmission rate than other formulations due to the lesser concentration of HPMCE15 & with higher concentration of EC.
For flatness determination, one strip is cut from the centre and two from each side of patches. The length of each strip is measured and variation in length is measured by determining percent constriction. Zero percent constriction is equivalent to 100 percent flatness. All the formulation shows 100% flatness and this indicates no amount of constriction in formulated transdermal matrix patches.
Estimation of drug content is essential to check the content uniformity of different patches from a single batch. The drug content was found in the range of 92.41 to 95.9 %. The drug content (DC) analysis of the patches has showed that the process employed to prepared patches was capable of giving uniform DC and minimum batch variability. The values for drug content of each formulation are given in the table.
The surface morphologies of the patches (blank and drug containing patches) were investigated by using a JEOL, JSM-6360 scanning electron microscope at 7 kv. It was observed that the picture of SEM of blank patch is very clear and drug is uniformly distributed in the picture of SEM of drug containing patch.
Conducting a drug release study of the patch is essential to ensure the drug concentration at the surface of stratum corneum is greater than the drug concentration in the body to achieve a constant rate of permeation through diffusion. Drug release mechanisms and kinetics are two characteristics of the dosage forms which play an important role in describing the drug dissolution profile from a controlled release dosage form. A number of mathematical model have been developed to describe the drug dissolution kinetics from controlled release drug delivery system e.g., Higuchi, First order, Zero order model. The dissolution data is fitted to these models and the best fit is obtained to describe the release mechanism of the drug.
All the five formulations studied and data was fitted to mathematical model Zero order, First order, Higuchi to explain the release mechanism and pattern. The profiles are plotted between the cumulative amounts of drug released as a function of square root time which fits better with the linear regression than the other plots. The coefficient of correlation was calculated are shown in Table 4.
It clearly reveals that release of Ondansetron HCl increases when the ratio of HPMC E15 in the patch is increased and Ondansetron HCl release decreases when the ratio of EC in patch is increased. The water uptake or absorption behaviour of the polymeric patch plays an important role at the beginning stage of drug release from dosage form. Thus, the patch with higher moisture uptake (formulations having more HPMC E15) supposed to give higher drug release rate. Initial rapid release was observed, gradually approaching constant value for the rest of time, thus confirming to the controlled release behavior of the formulations. The initial quick release (burst effect) would help to achieve the therapeutic plasma concentration of the drug in minimum time. The formulation having higher ratio of HPMC E15 in formulation OH 1 and OH 2 shows cumulative % drug release 89.10 and 77.12 respectively in one hr. Whereas, in case of formulation having lower ratio of HPMC E15 in formulation (OH 3, OH 4 and OH 5) shows lower cumulative % drug release 59.66, 51.74 and 43.30 respectively in one hr.
The in-vitro permeation study was done to see the effect of polymer ratio on permeation of drug through dialysis membrane from patch having polymer ethyl cellulose and hydroxypropylmethyl cellulose E15 in different concentration to optimize the formulation for ex vivo study. All the five formulations were studied and data was fitted to different mathematical models Zero order, First order and Higuchi to explain the diffusion mechanism and pattern. The study clearly showed that Ondansetron HCl permeation decreased when the ratio of EC in the patch increased. The % cumulative permeation, calculated over the study time range of 0–6 h, of Ondansetron HCl patches prepared with HPMC E15 / EC ratio of 2:1, 3:2, 1:1, 2:3 and 1:2 are shows maximum % cumulative permeation approximately 44.2%, 32.55%,31.85%,29.02%,28.26% respectively.
The present research was carried out to develop matrix type transdermal therapeutic systems of Ondansetron hydrochloride. The characterization of physicochemical properties of the prepared transdermal drug delivery system of Ondansetron hydrochloride using two polymers such as HPMC E15 and EC in different ratio had shown that the formulations are physic-chemically stable including the absence of drug-polymer interaction, which was ascertained by FTIR study.
The release rate of Ondansetron hydrochloride from the polymeric matrix patches can be varied by selecting appropriate ratio of hydrophilic (HPMC E15) and hydrophobic (EC) polymers. Usually the release rate as well as the rate of permeation can be retarded to get sustained release characteristic of the formulation by incorporating higher proportion of hydrophobic (EC) polymer.
Matrix patches showed an initial burst effect to provide the loading dose of the drug, followed by sustained release, indicating a promising potential of the Ondansetron hydrochloride matrix patches as an alternative to the conventional dosage form. Further work is recommended in support of its efficacy claims by long term pharmacokinetic and pharmacodynamic studies on human beings.