ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ch V Satya*, C. Anuradha, D. Sri Rami Reddy
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
L-asparaginase is one of the known drugs in the treatment of cancer especially acute lymphoblatic leukemia. In this study, the production of high levels of L-asparaginase was obtained from Aspergillus wentiiin solid state fermentation using agro industrial wastes like coconut oil, palm oil, white and black sesame oil cakes. Palm oil cake showed maximum enzyme production with Aspergillus wentii when used as the substrate source in solid state fermentation (SSF). A 48 h fermentation time under aerobic conditions with moisture content of 89.51% and inoculum volume 30% V/W in SSF appeared optimal for enzyme production. The optimum temperature and pH for enzyme activity were 34oC and 5.8 respectively.
Keywords |
| Solid state fermentation, Palm oil cake, Asparaginase, Aspergillus wentii |
I. INTRODUCTION |
| L-asparaginase attracted much attention because of its use as effective therapeutic agent against lymphocytic leukemia and other kinds of cancer in man (1). Cancer cells differentiate themselves from normal cells in diminished expression of Lasparagine (2, 3). Hence, they are not capable of producing L-asparagine, and mainly depend on the L-asparagine from the circulating plasma pools (2). |
| L-asparaginase production using microbial systems has attracted considerable attention, owing to the costeffective and eco-friendly nature. A wide range of microorganisms such as filamentous fungi, yeasts, and bacteria have proved to be beneficial sources of this enzyme (4, 5, 6, 7, 8) |
| The SmF technique is a cost intensive, highly problematic, and poorly understood unit operation (9). SSF offers numerous advantages over submerged fermentation (SmF). SSF should not be seen as a technology, which can simply replace SmF. Solid-state fermentation is a very effective technique as the yield of the product is many times higher when compared to that in SmF(10), and it also offers many other advantages (11). |
| The objective of this study was to produce L-asparaginase from Aspergillus wentii with palm oil cake by solid state fermentation. |
II. MATERIALS AND METHODS |
| Microorganism and cultivation conditions: Aspergillus wentii NCIM 941 procured from National Collection of Industrial Microorganism (NCIM), Pune, wasmaintained on potato dextrose agar medium, incubated at 370C, stored in refrigerator and subcultured monthly. |
| Preparation of seed medium |
| Conidial suspension was prepared from a freshly raised four day old culture of Aspergillus wentii and inoculated to100 ml of asparagines-dextrose-salts broth that contained L-asparagine (1.0%), dextrose (0.2%), kH2PO4(0.05%), MgSO4.7H2O(0.01%) and pH was adjusted to 7.2 (12). Substrates: Locally available coconut oil cake, palm oil cake, black sesame oil cake and white sesame oil cake were obtained from M/S Laxmi Durga Enterprises Oil mill and grounded. The ground material was collected and used as substrate in SSF. |
| Enzyme production by Solid State Fermentation |
| Five grams of the oil cake was taken in 250 ml Erlenmeyer flasks and moistened with 4 ml 0.01M phosphate buffer (pH 6.5). The thoroughly mixed fermentation media was sterilized by autoclaving for 15 min at 15 psi and 1210C and then inoculated with 1 ml of the previously prepared fungal suspension at 370C for desired time period.For the extraction of crude enzyme, weighed quantity of fermented substrate was mixed with phosphate buffer (1:5) (13,14). The mixture was homogenized, filtered and the filtrate was centrifuged and clear supernatant was used for enzyme assay. |
| Quantitative assay for the estimation of L-asparaginase Activity: |
| To determine the enzyme activity, 5mL of the culture broth was withdrawn aseptically from the flasks at an interval of every 24h. The broth was filtered using Whatmann filter paper no.1 and then centrifuged at 9000 rpm for 8min(14). The supernatant thus obtained was used as crude extract for L-asparaginase assay. Assay of enzyme was carried out as per Imada et al.(15). |
| International Unit |
| The enzyme activity was expressed in IU. One IU of L-asparaginase is the amount of enzyme which liberates 1μmole of ammonia per mL per min(μmole/mL/min) |
III. RESULTS AND DISCUSSION |
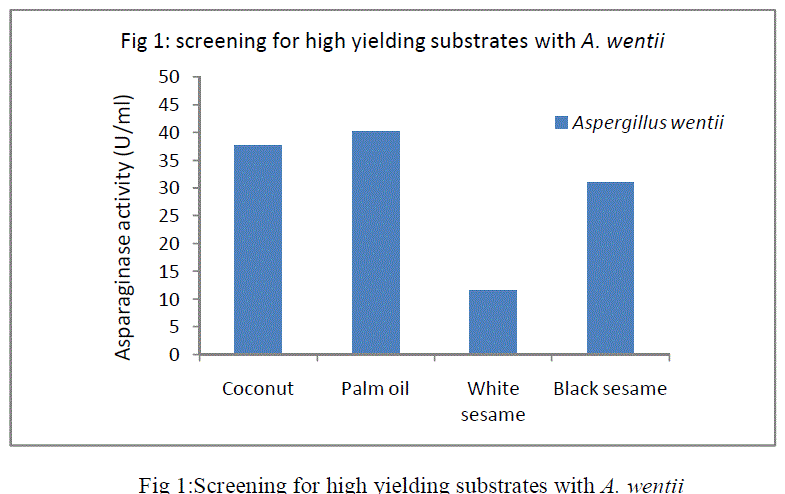
| In SSF, the selection of a suitable substrate for the fermentation process is a critical factor. In the present study, among the four substrates screened (Fig 1 )palm oil cake with A. wentii gave highest enzyme production. The optimization studies of the fermentation parameters were carried out using palm oil cake as the substrate for the production of L-asparaginase. |
 |
| Effect of Incubation time, moisture content, Temperature, pH on L-asparaginase production by |
| Aspergillus species |
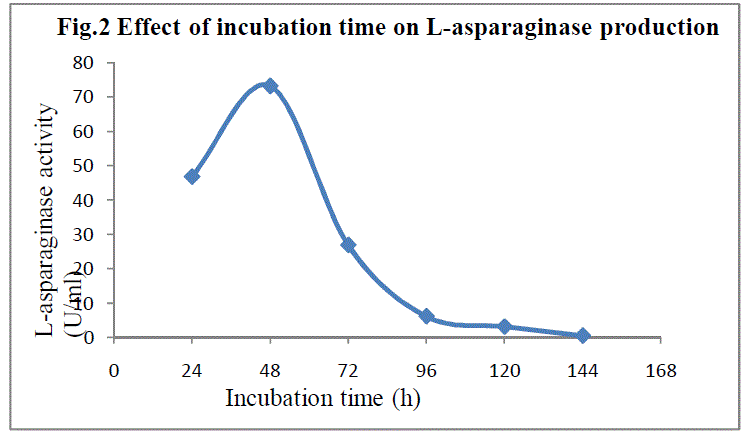
| SSF was carried out for a period of 144h. The fermented flasks were taken at every 24h and the enzyme extraction was done as described earlier.The enzyme production showed growth relatedness as the fermentation time progressed and the maximum enzyme production(73.251 IU) was observed after 48h.(Fig 2).Similar observations were reported by Alberdon Silva Santos et al13 for the production of L-asparaginase by Aspergillus terreus at 48th h. |
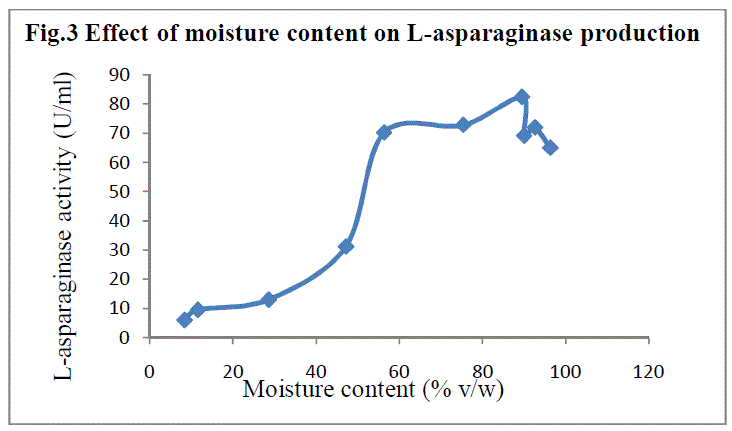
| Production medium with different moisture content was used to determine its influence on asparaginase production. Maximum enzyme yield was observed at 89.51% (v/w) with 82.353 IU of enzyme activity(Fig 3). Moisture content is a critical factor in SSF since the moisture of the medium determines the microbial growth and enzyme yield (16) |
| Incubation temperature is another important factor, which affects the enzyme production in SSF. Maximal enzyme production of 84.954 IU was recorded at 34oC and production was reduced at temperatures higher than 34oC(Fig 4). |
| The pH influence on L-asparaginase activity was studied using 0.1 M phosphate buffer at different pH values of 5.7-8.0 and maximum enzyme activity 90.025 IU was obtained with pH 5.8, with a gradual decrease at lower pH value, as well as a sharp decline at higher values (Fig 5). pH strongly influence many enzymatic processes and transports the cell growth and product production.Similar observations were reported by Abha Mishra17 with pH 6.5 for the production of L-asparaginase fromAspergillus niger under solid state fermentation. |
 |
 |
IV. CONCLUSION |
| In the present investigation, the maximum value of L-asparaginase activity obtained after the optimization of all the fermentation parameters was (89.954 IU), which clearly demonstrated the scope for exploring filamentous fungi for the production of therapeutic enzymes like L-asparginase using SSF. |
| L-asparaginase can be produced from low-cost untreated biomass residues without addition of supplements to enhance growth or production. SSF revealed the possibilities of effective utilization of oil cakes for value addition through biotechnological means. These processes help in reducing the outlay of production. |
| The study suggests that choosing an appropriate substrate and organism when coupled with process level optimization improves enzyme production markedly. Developing an asparaginase production process based on palm oil cake as a substrate using Aspergillus wentii in SSF is economically attractive as it is a cheap and readily available raw material in agriculture based countries. |
ACKNOWLEDGEMENT |
| Author Ch V Satya is thankful to Department of Science and Technology, New Delhi for financial assistance under Women Scientist (WOS-A) Scheme. |
References |
|