ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Patil Basavani K.1, Rathod Sopan M.2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Cox Ni1-x Fe2 O4 (x= 0.5, 0.7, 0.9) nanoferrite powder were prepared using a simple cost effective, solgel autocombustion method at low temperature. Metal nitrates are used analytical grade, such as Nickel nitrate, Cobalt nitrate and ferric nitrate, were used as the source materials. Citric acid was used as the burning agent and the metal nitrate- to -citric acid taken as ratio 1:3. The pH of the precursor was maintained at 7. The synthesized powder was characterized by TGA/DTA it shows that the phase formation, The FTIR spectroscopy is used to deduce the structural investigation and confirmation of ferrite, The X-ray diffraction (XRD) was used to determine the particle size and structural properties. The Vibrating Sample Magnetometer (VSM) was used to obtain the Hysteresis parameters. The magnetic property of the samples shows remarkable changes with change of Co 2+. The variation of Co2+ substitution has a significant influence on the grain size and magnetic properties. The mean crystalline size of the prepared ferrite was in the range of ~ 36 - ~45 nm.
Keywords |
| Sol-Gel, Co-Ni nanoferrite, FTIR, XRD and VSM |
INTRODUCTION |
| Ferrite nanoparticles are of great interest because of their scientific aspect and various applications. The nanoferrites are interesting materials owing to their wide range of applications in modern science and technology [1]. They have recently attracted considerable research interest on their structural, magnetic and electrical properties [2]. These structures are attractive for microwave applications, magnetic sensors and catalytic materials owing to their great magnetic permeability and dielectric constant, low dielectric loss, high Curie temperature as well as mechanical strength and chemical stability at low frequencies [3]. In addition, their magnetic properties can be controlled and tailored to practical applications through the appropriate choice from a number of divalent cations in their structure [4]. Ni 2 + substituted Mg-Zn spinel ferrite has been synthesized by sol-gel method and studied phase crystal structure and morphology [5]. Cu2+ ion concentration in Ni-Zn spinel ferrite by combustion method has been investigated single phase cubic structure and surface morphology. [6]. Permanent magnets, targeted drug delivery and high density information storage devices. From crystal structure point of view, ferrites are generally divided into two groups: cubic or spinel ferrites and hexagonal or hexaferrites [7]. |
| Cobalt ferrite is a well known hard magnetic material with a high coercivity and a moderate magnetization. These properties along with its great physical and chemical stabilities, make CoFe2O4 nanoparticles suitable for many practical applications such as audio/ video tapes, high density digital recording disks, etc.[8]. On other hand Nickel ferrite is a typical soft magnetic material [9], which has many applications in electronic devices, such as inductors and transformator cores [10]. Ni substituted cobalt ferrite nanoparticles were prepared by sol-gel method. The crystallite size and lattice parameter studied in the range of 120-70nm and 8.350 – 8.300 respectively. [11]. Nickel substituted cobalt ferrites are highly resistive and magnetostrictive. Studies of Van Uitert and Jilg showed that a very large increase at room temperature respectively of nickel ferrite is achieved by substituting 1or 2 percent of cobalt ions.[12]. |
| Particles with nanosize exhibit unique chemical and physical properties. In particular nano composite material composed of nanometric metal and metal oxide particles embedded in vitreous matrices reveal a variety of interesting magnetic, electric and catalytic properties. Ferrites are ferrimagnetic semiconductors that opened a new area in the physics of material science and the needful high resistivity ferrites led to synthesis of various ferrites. The electrical and magnetic properties of ferrites depend on the method of preparation [13], Magnetic properties of magnetic nano materials particularly in ferrites materials also depend on their chemical composition and methods of synthesis [14]. The substitution effect and the change of the preparation condition are allowed to improve the properties of ferrites. Generally ferrites were commercially used in radio frequency circuits, transformer cores, antennas and for high speed digital tape. Nickel substituted cobalt ferrite nanoparticles are studied and reported the particle size decrease from 8350A to 8300A with increasing nickel contents[15]. Cobalt ion containing nickel ferrite are studied gas response on nickel ferrite by using sol-gel autocombustion method. The hkl values are exactly same with our results[16]. |
| In this investigation, the effect of Co2+ substitution in Ni Fe2O4 is studied. The sol-gel method is used to synthesize the nanoparticles of CoxNi1-xFe2O4. The structural, optical and magnetic properties of the synthesized samples have been discussed in the contents. |
EXPERIMENTAL METHOD AND MATERIALS |
| Co-Ni ferrite powders were synthesized by sol-gel autocombustion technique at low temperatures for different compositions Co.x Ni1-x Fe2O4 (where x= 0.5, 0.7, 0.9,). Raw materials are used in the experiments are AR grade nitrates i.e. Ni(NO3)2, Co(NO3)2, Fe2(NO3)2and C6H8O7 is used as a fuel and the metal nitrate- to -citric acid taken as ratio 1:3 all from Merck co. of purity of 99 % using stoichiometric ratio and dissolved in distilled water. The mixture of the raw material was stirrered at 80 0C on hot plate magneto-stirrer. Maintaining pH 7, it was continuously stirrered to obtain uniform gel. After 4-5 hours it converts from gel to ash form, which was sintered at 560 0C. The FTIR characterization shows the bond formation and synthesized material is ferrite. The TGA_DTA characteristics have been taken to determine the temperature range for growth of these systems Differential Thermal Analysis and Thermo Gravimetric Analysis in temperature range 00C -8000C was performed. The structural and average grain size is studied by X-ray diffraction (XRD), it is in crystal nature and average particle size is~ 45 to ~15 nm. Particle size decreases with increasing the percentage of Co2+. Also lattice constant, (hkl) planes and grain size was calculated by Bragg’s law and Scherer’s formulae. From VSM the magnetic properties of the samples show remarkable changes with change of Co2+ percentage. |
RESULTS AND DISCUSION |
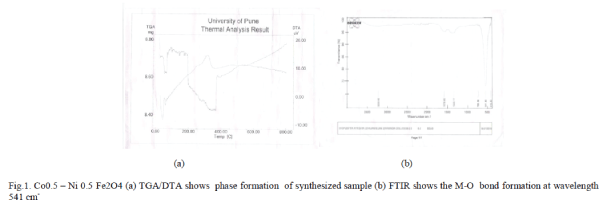
| I. TGA-DTA: (Thermo Gravimetric Analysis and Differential Thermal Analysis): The TGA-DTA has been taken of the sample to determine the temperature range for growth of these systems Thermo Gravimetric Analysis Differential Thermal Analysis and in the temperature range 00C -8000C was performed. Figure 1(a) shows the TGADTA graph of Co.x Ni1-xFe2O4. It may be noted that the transformation from precursor powder to final phase is accompanied 5600C. The very first weight loss around 1000C can be attributed to vaporization of water molecules from surface and then at 200 0C from the trapped water. In order to decompose the citrate network, higher temperature is needed and that is evident from the weight loss at 300 0C. The conversion process starts at around 400 0C and finally get converged into the well grown ferrite particles at a temperature 560 0C. The thermogravimetric analysis goes in sync with the DTA curve as well where we see a significant endothermic peak. This gives an indication that the ferrite formation get completed at a temperature around 560 0C. It shows the phase formation at 5600C. |
II. FTIR Spectroscopy: (Fourier Transform Infrared Spectrometer) |
| The FTIR ( Fourier Transform Infrared Spectrometer) characterization from figure 1shows the bond formation and two main metal – oxygen bonds at 541 cm-1 so that it is conform that the synthesized material is ferrite. |
III. XRD characterization: |
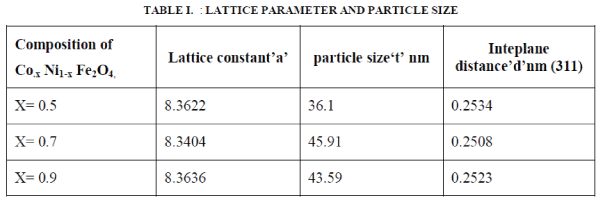
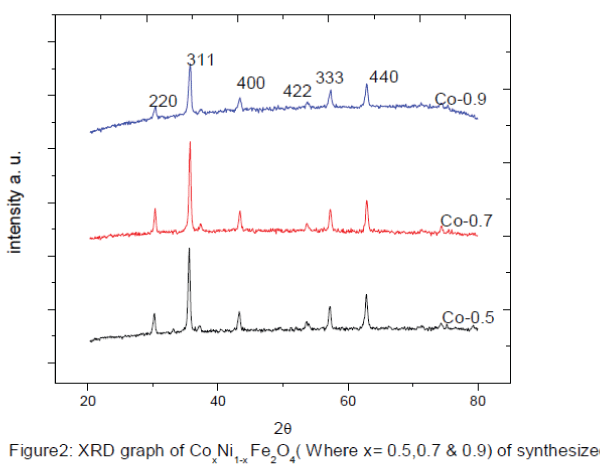
| Figure 2 shows the X-ray diffraction (XRD) patterns of typical samples of Co.x Ni1-x Fe2O4 (where x= 0.5, 0.7, 0.9,). The XRD patterns shows well developed diffraction line assigned to pure inverse spinel phase. The all measured XRD peaks match well with the standard patterns of inverse spinel ferrite. Interplane distance and planes are calculated by Bragg’s diffraction law and index method using equation 1, and 2. The nanoparticles exhibit several diffraction peaks which can be indexed as cubic structure. The average crystalline size of the prepared Co.x Ni1-x Fe2O4 nanoparticles was found in the range of ~ 36 - ~45 nm by using Sherrer’s formula equation 3and results are given in table 1. The intensity & width of the Bragg’s peak Conforming, good crystallinity & nano particle size. The interplane distance were calculated by using bragg’s equation from the relation |
| The lattice parameter ‘a’ was calculated using following relation |
| Where (hkl) is the miller indices, λ is the wavelength of X-ray radiation and θ is the Bragg angle. The results of interplane distance and lattice parameter are shown in table 1. |
The particle size were calculated using Scherrer’s formula  |
| Where‘t’ is the particle size, and β is the FWHM (full width half maxima) of the peak θ |
 |
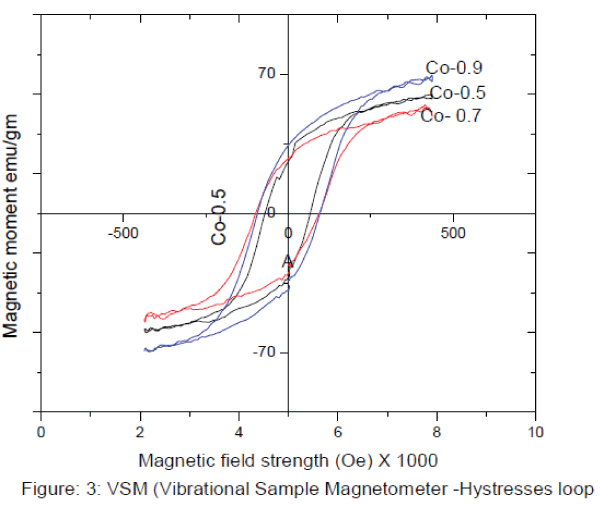
IV. VSM (vibrating sample magnetometer) CHARECTERIZATION (Hysteresis loop) : |
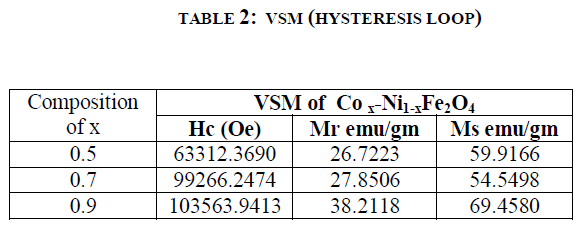
| VSM (vibrating sample magnetometer) CHARECTERIZATION (Hysteresis loop) : Hysteresis studies:The different parameters such as saturation magnetization (Ms), coercive force (Hc), and retentivity (Mr) The figure 3 shows the magnetic properties of the synthesised samples, it shows that the compositions of Co2+ containt increase the Hc (Coercievity),and Mr (Magnetic remenance) are the increases but Ms (Magnetic saturation) decrease at x=0.7 and again it increase at x=0.9. The results are illustrated in table 2. |
 |
CONCLUSION |
| The Co.x Ni1-x Fe2O4 (where x= 0.5, 0.7, 0.9,). nanoferrites were synthesized using sol-gel technique. The increase in the Co2+ concentration gives the significant changes in magnetic properties of the composition Co.x Ni1-x Fe2O4 (where x= 0.5, 0.7, 0.9,). The particle size is obtained 36 to 45 nm. The FT-IR spectroscopy study shows two main metal oxygen bonds at in the range of 500-600 cm-1confirming the formation of single phase cubic inverse structure of Co2+ substitute Ni ferrite. The crystalline particle size was found that in the range of ~ 36 - ~45 nm. The lattice parameter decreases with increasing Co2+ in Ni. From the hysteresis loop, it is clear that the synthesized sample can be control, for the Co2+ substituted in Ni ferrite at the percentage of 50 it is soft and 70 and 90 it goes to hard ferrite. |
 |
 |
 |
ACKNOWLEDGMENT |
| We thankful to the Department of Physics & Chemistry, University of Pune. We are also thankful to the Department of Chemistry, Nowrosjee Wadia College, Pune, and Department of Physics, Abasaheb Garware College, for their kind cooperation and providing their infrastructure for the characterization purpose. |
References |
|