ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Sreehari Sastry1,* S.Vedavyas2, B. Rupa Venkateswara Rao3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Mn(II) doped P2O5-ZnO-CaO glasses are being characterized by techniques like XRD, UVVisible and different physical properties. XRD patterns in present glasses have confirmed the amorphous nature of glass samples. The optical absorption spectrum exhibited three bands which are characteristic of Mn(II) in distorted octahedral site symmetry., The density is one of the tools to reveal the degree of structural changes of the glass network with composition. The higher value of optical basicity parameter for PMn1is observed, giving an indication to use the glass sample to design novel optical functional materials with higher optical performance
Keywords |
| Glass, XRD, UV-VIS, Tauc’s plots, density and optical basicity. |
INTRODUCTION |
| Due to the superior physical properties such as high thermal expansion coefficients, low melting, softening temperatures and high ultra-violet transmission phosphate glasses are more advantageous than conventional silicate and borate glasses [1, 2]. However the poor chemical durability, high hygroscopic and volatile nature of phosphate glasses have restricted their use in replacing the conventional glasses for a enhanced range of technological applications. The physical properties and chemical durability of phosphate glasses are found to improve by introducing of a number of heavy metal oxides into P2O5 glass network [3, 4]. Among various phosphate glass systems, alkali earth zinc phosphate glass systems are proven as more stable against devitrification and moisture resistant. Glasses containing transition metals like Cr3+, Mn2+ posses better semiconducting properties and hence these are used for several applications such as memory switching, electrical threshold [5-7]. Among all transition metal ions, manganese (Mn) ion is particularly interesting because it exists in different valence states in different glass matrices [8-10]. Manganese (55Mn) ions have been frequently used as paramagnetic probes to explore structure and properties of vitreous systems, as manganese ions have a strong influence on optical and magnetic properties of glass. A large number of studies are available on the environment of manganese ion in various inorganic glass systems [11-13]. Physical properties of glasses to a large extent are controlled by composition, structure and nature of bonds of glasses. Investigation over changes in physical properties of glasses on a controlled variation of chemical composition and transition metal ions is of considerable interest in the application point of view [14]. In order to study structural changes induced by MnO in phosphate glass network, Mn(II) doped P2O5-ZnO-CaO glass system has been prepared and investigated by means of physical properties, XRD, Tauc’s plots and optical absorption spectroscopy. |
EXPERIMENTAL METHODS |
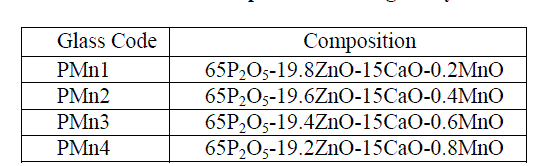
| Glass samples were prepared by conventional melt-quench technique. The starting materials used were P2O5, ZnO, CaO and MnO of analytical reagent grade. These chemicals were thoroughly mixed and grounded for 40 minutrd in a mortar pastel and melted in a porcelain crucible placing high temperature electric furnace for 5 hours between the temperature range 800-1100oC depending on composition. When the melt was thoroughly homogenized and attained desirable viscosity the material was poured on a metal plate. Prepared glass was annealed at a temperature (300°C) for 2 hrs and stored in desiccators prior to characterization. X-ray diffraction patterns were recorded on powdered samples at room temperature using Philips X-ray diffractometer. Optical absorption spectra of these glasses were recorded using JASCO model V-670 UV-VIS-NIR spectrophotometer. Composition of the glass samples are given in Table 1. |
 |
RESULTS AND DISCUSSIONS |
| Density of amorphous material is mostly the simplest physical property and highly informative if structure of material could be well defined. The Change in atomic geometrical configuration, co-ordination number, cross-link density and dimensions of the interstitial space in glass network determine the density. Hence, density is a tool to reveal the degree of change in the glass structure with composition [18]. Jen and Kalinowski [19] reported a model for describing the bridging to non-bridging oxygen ratio as a function of the glass composition. Average molecular weight is always proportional to density. In present investigation, the average molecular weight has decreased from PMn1to PMn4 glass sample and also density has decreased in a similar way.. But molar volume has increased with the increase of MnO content in the glasses. The trends in density and molar volume with MnO content are shown in Fig.1. |
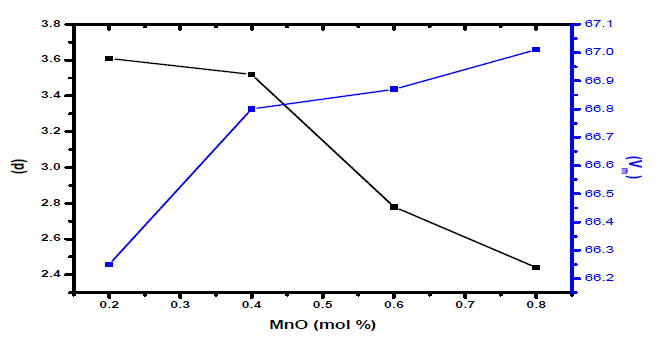 |
| Glass structure has been explained in terms of molar volume rather than density, as the former dealing the spatial distribution of the ions that form structure. Change in molar volume with molar composition has indicated the preceding structural changes that are due to formation or modification process in glass network. Density of the glasses has decreased considerably with the MnO content. Structural compactness, modification of geometrical configuration of glassy network, and changes in coordination of glass forming ions are the principal factors responsible for observed density variability. |
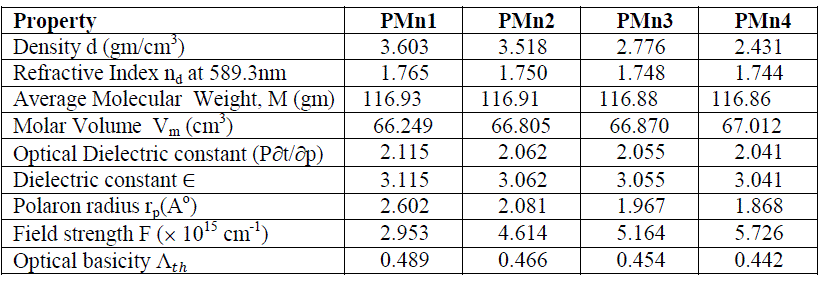
| The data in Table 2, have shown that decrease in average molecular weight M has significantly influenced both refractive index and density and besides other physical properties. The theoretical value of optical basicity (ïÃÂÃÅth ) has slightly changed from PMn1 glass to PMn4 glass. The theoretical value of optical basicity (ïÃÂÃÅth) is shows the ability of glass to donate negative charges to probe ion. High optical basicity means high electron donor ability of oxide ions to cations [20]. Polaron radius (rp) values have deceased with the increase of manganese content, however field strength has shown quite reverse trend. So these two parameters have shown the change of environment in the present glass system. From the data presented in Table 2, it can also be seen that, other observations drawn from the Table is that the physical properties are changing from glass to glass and hence, the environment around the Mn+2 ions in these glasses also changes with composition. |
| In phosphate glasses, the calcium oxide introduced acts as modifier oxide. Alkaline earth oxides added have been responsible for formation of non-bridging oxygen ions (NBOs) in phosphate matrix. This type of ions represents broken bonds in the network. The calcium ions are linked to the surrounding oxygens by bonds which are much more ionic that are weaker than P-O bond. |
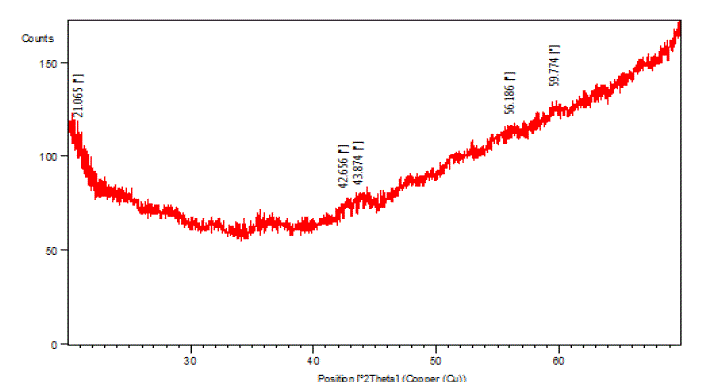
 |
| The above figure (Fig. 2) shows the XRD patterns of present glass system. It has a broad diffuse scattering at different angles instead of crystalline peaks and no continuous or discrete sharp peak. This reflects the amorphous characteristic of glass samples. |
 |
| The Mn2+ ion has five unpaired electrons in the valence shell distributed in the t2g and eg orbitals either in Oh or Td, symmetry. In free ion state it will give rise to a number of free ion terms in the increasing order of energy 6S, 4G, 4P, 4D, 2I, 2G, 2H, 4F, 2D, 2F, 2F, 2S, 2D, 2G, 2P and 2D [21]. Fig. 3 has presented optical absorption spectra of glasses in the wavelength region of 280-600 nm. The absorption spectra have exhibited three weak bands which are centred at about 402, 448 and 555 nm. On increase of MnO content, all these bands have been gradually shifting towards red spectral regions. Optical absorption bands observed have originated from the ground state 6A1g to some quartet states. These are both spin and parity forbidden. Using Tanabe Sugano diagram for d5 electron, the bands observed originated from 6A1g (S) → 4Eg(D), 4T1g (G), 4T2g (G) octahedral transitions of Mn2+ ions, respectively [22]. The bands are sharp as these arise from intra-configurational transitions. The optical absorption spectral studies revealed the existence of manganese ions in Mn2+ state [9, 23]. The type of glasses is very much useful in technological importance like electronic, tunable solid state lasers and fiber optic communication systems [24]. |
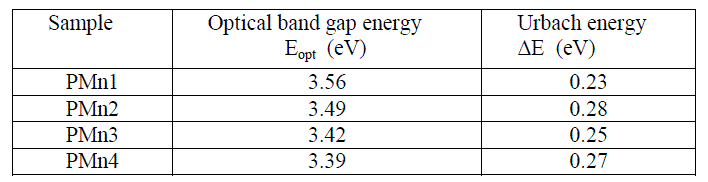 |
| Tauc’s or Urbach energy plots of the samples studied are presented in Fig.4. The optical band gap values as shown in Table 3 are varied with change of concentration of manganese content from 3.39 to 3.58 eV. Urbach energy values are present in between 0.23 and 0.36 eV. Smaller values of Urbach energy indicated that Mn2+ doped phosphate glasses are homogeneous and stable [25, 26]. In present study PMn1 glass has least value of Urbach energy. It informs PMn1 is the most stable and homogeneous glass and having minimum defects than PMn2, PMn3 and PMn4 glasses [27]. |
CONCLUSION |
| Physical properties of these glasses indicated the environment around the Mn+2 ions that has changed from PMn1glass to Pmn4 glass. The increase in molar volume value is related to bond length or inter atomic spacing. Change in refractive index is attributed to the formation of NBO. The shift of absorption peak in UV-visible spectra revealed the compositional dependence of the glass. Clearly this is due to Mn2+ occupying an Oh site in the glass network. Tauc’s plots of the samples reveal that PMn1 glass is most stable and homogeneous than remaining glasses. High optical basicity of PMn1 is the indication for incorporation of novel optical functional materials for higher optical performances. |
ACKNOWLEDGMENTS |
| The authors gratefully acknowledge UGC DRS LEVEL III program No.F.530/1/DRS/2009 (SAP-I), dated 09-02-2009 and DST FIST program No DST/FST/ PSI – 002/2011dated 20-12-2011, New Delhi, to the department of Physics, ANU for providing financial assistance. |
References |
|