ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Mrudula C M 1, Ashish A Prabhu2, RituRaval*3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present study was carried out to elucidate free radical scavenging activity of the leaves of locally grown trees of Moringaoleifera, Basellaalba and Centellaasiatica. The total polyphenol, flavonoids, tannins, antioxidant capacityand the free radical scavenging activity were estimated. The free radical scavenging activity of the leaves of M. oleifera, B. alba and C. asiatica were assayed by using .2, 2-diphenyl-1-picrylhydrazyl (DPPH). It was found that C. asiatica showed the highest amount of phytochemicals (2.5% polyphenols, 0.741%flavonoids and 8% tannins) and also exhibited excellent antioxidant capacity and radical scavenging ability which was determined by the IC50 values. M. oleifera also exhibited comparable results as that of C. asiatica. B. alba did not prove to be a very good scavenger in comparison to the other two leaf sources. The results indicate that C. asiatica leaves could be an important source of natural radical scavengers.
Keywords |
| Moringaoleifera, Basellaalba, Centellaasiatica, polyphenols, flavonoids, tannins, radical scavenging activity |
INTRODUCTION |
| Diet and health status relationship is well established by epidemiological and clinical studies. Consumption of a large proportion of plant-based foods, including fruits, vegetables, whole grains and cereals, have a lower incidence of chronic and acute disorders. Therefore, interest has been expressed in functional foods,nutraceuticals and dietary supplements [1].Nutraceuticals are food or part of a food that provides medical or health benefits, including the prevention and/or treatment of a disease. In other words, it provides medical or health benefits and helps in the prevention and treatment of diseases [2]. Plant foods contain thousands of natural chemicals, also called phytonutrients or phytochemicals. Phytochemicals are present in virtually all of the fruits, leaves, vegetables, legumes (beans and peas), and grains we eat, so it is quite easy for most people to include them in their diet [3]. Researchers have come up with the fact that some plant chemicals which have been regarded as anti-nutritional or anti- nutrients have potentials in helping to reduce the risk of several deadly diseases in man [4]. Several of the nutrients in plants have known potential for reducing risk factors for chronic disorders; the linoleic acid, fiber, vitamin-E, Selenium and folate. Plants also contain several phenolic acids with antioxidant properties [5]. Many plants have been traditionally used in the treatment of diabetes. Polyphenols contained in these plants may explain some of their therapeutic activity [6].It has been demonstrated that dietary polyphenols have other bioactive effects, such as antibacterial activity, anti-HIV effect, hepatoprotective ability, and angiogenesis effect [7]. Dietary polyphenols represent a wide variety of compounds that occur in fruits, vegetables, wine, tea, extra virgin olive oil, chocolate and other cocoa products. They are subdivided into groups by the number of phenolic rings and of the structural elements that link these rings: (1) The phenolic acids with the subclasses derived from hydroxybenzoic acids such as gallic acid and from hydroxycinnamic acid, containing caffeic, ferulic, and coumaric acid; (2) the large flavonoid subclass, which includes the flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols; (3) the stilbenes; and (4) the lignans and the polymeric lignins[8]. The major active nutraceutical ingredients in plants are flavonoids. As is typical for phenolic compounds, they can act as potent antioxidants and metal chelators. They also have long been recognized to possess anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities [9]. Flavonoids are powerful antioxidants against free radicals and are described as free-radical scavengers. This activity is attributed to their hydrogen-donating ability [10]. Tannins are polyphenolic secondary metabolites of higher plants. In nature the tannins are found worldwide in many different families of the higher plants; depending on their origin, their chemistry varies widely, having a molar mass of up to 20000 Daltons. High tannin concentrations are found in nearly every part of the plant, such as in the bark, wood, leaves, fruit, roots, and seed [11]. Different isolated hydrolysable tannins from edible and/or non-edible plants have shown strong biological activity in the form of anti-tumors, anti-mutagenic, anti-diabetic, anti-proliferative, and anti-microbial properties [12]. Plants based natural constituents can be derived from any part of plant like bark, leaves, flowers, roots, fruits, seeds, etc i.e. any part of the plant may contain active components . The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant [13]. It has been reported that several phyto chemicals and antioxidants are found in leaves as secondary metabolic compounds [14, 15, 16, 17 ]. The objective of the current study was to quantify and estimate Phytonutrients in three different leaf samples; M. oleifera, B. albaandC. asiatica. The phytoonutrients studied include polyphenols (soluble), flavonoids and tannins (hydrolysable). Antioxidant activity of the plant sources was assessed and the IC50 values for free radical scavenging assays were calculated. The three leaf sources were compared for phytochemical screening. |
II MATERIALS AND METHODS |
| A. Materials Gallic acid, Methanol, Hydrochloric acid (HCl), Folinciocalteau reagent, sodium carbonate (Na2CO3), Sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium hydroxide (NaOH), ferric chloride (FeCl3) and Phosphomolybdenum reagent were procured from Sisco Research Lab, Mumbai, India. Catechin was purchased from Sigma-Aldrich and Potassium ferricyanide (K3Fe(CN)6) from Loba chemicals. 2, 2-diphenyl-1-picrylhydrazyl (DPPH+) was procured from Merck chemicals and tannic acid from Himedia. B. Plant material collection and processing Leaves of M. oleiferaandB.albawas purchased from local market Udupi. C. asiatica was procured from local house garden in Bhadravathi, Shimoga, Karnataka. Fresh plant material was washed under running tap water, sun dried, and then ground to fine powder and stored in airtight bottles [18]. C. Sample extraction Samplewas extracted according to the procedure given by [18]. Ten grams of dried plant material was extracted with 100 ml of methanol kept on a rotary shaker for 24 h. Thereafter, it was filtered and centrifuged at 5000 g for 15 min. The supernatant was collected and the solvent was evaporated to make the final volume one-fifth of the original volume. It was stored at 4ºC in airtight bottles for further studies. The above extracts were used to quantify polyphenols, flavonoids, tannins and total antioxidant capacity. For DPPH+ free radical scavenging activity assay and hydroxyl radical scavenging activity assay, 30 g of the powder was then extracted with 300 ml of methanol, filtered, squeezed off and evaporated under reduced pressure in a rotary evaporator to obtain crude extract [19]. D. Total polyphenolic content (TPC) Total polyphenol content (soluble and bound) was determined by the method described by Singleton and Rossi (1965) [20]. Known volume of extract was taken and made up to 250μl with distilled water and the sample was mixed with 250 μl of folinciocalteau reagent and 500 μl of 20% Na2Co3. The final reaction mixture volume was adjusted to 5ml using distilled water. The reaction mixture was incubated in dark for 30 minutes and samples were then centrifuged at 2000 rpm for 5 minutes after which the supernatant absorbance was measured at 760 nm. A calibration curve was constructed with different concentrations of gallic acid as standard. The results were expressed as mg of gallic acid equivalent/100g of sample. E. Total flavonoids content This was assayed as described by Jiaet al. (1999) [21] with slight modification. A known amount of above mentioned extract was taken and made up to 500μl with distilled water. To this mixture 75μl of 5% sodium nitrite was added and allowed to stand for 5 minutes after which 150 μl of 10% AlCl3 was added and after 6 minutes 0.5 ml of 1M NaOH were added and sample mixture was incubated at dark for 15 minutes. It was then centrifuged at 2500 rpm for 5 minutes and absorbance was recorded at 512nm. The flavanoid content was expressed as mg of catechin equivalent/100g of sample. F. Total Tannins content The quantitative tannin content in samples was estimated by the method of Price and Butler [22] with some modifications. Known concentration of methanolic extract were taken and made up to 0.5ml using distilled water. To this reaction mixture, 1 ml 1% K3Fe(CN)6 and 1ml 1% FeCl3 were added, and the volume was made up to 10 ml with distilled water. The reading of the resultant solution was measured spectrophotometrically at 720nm after 5 min using tannic acid as a standard. The tannin content was expressed as mg of tannic acid equivalent/100g of sample. G. Total antioxidant capacity assay (TAO) The total antioxidant capacity assay was determined as described by Prietoetal[23] with slight modification. Known concentration of methanolic extract were taken and 1ml of phosphomolybdenum reagent (0.6 M sulphuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). The tubes were capped and incubated in dry bath at 90ºC for 90 minutes. After cooling to room temperature, the absorbance of the aqueous solution was measured at 695nm against blank. Ascorbic acid was used as standard and total antioxidant capacity is expressed as equivalent of ascorbic acid. H. DPPH+ free radical scavenging activity assay The free radical scavenging activities of methanolic extracts of leaves were tested for their ability to scavenge the stable radical DPPH. The antioxidant activity using DPPH (2, 2-diphenyl-1-picrylhydrazyl assay was assessed as described by the method of Bondet et al, 1997 [24]. The reaction mixture contains 0.08 mg/ml DPPH in methanol and different concentration of leaves extract (0.5-3.5 mg/mL). Absorbance at 517nm was determined after 30 min incubation at room temperature and the scavenging activity were calculated as percentage of radical reduction using catechin as the reference compound. I. Hydroxyl radical scavenging activity assay The antioxidant activity using hydroxyl radical scavenging was assessed by the method of Smirnoff and Cumbes, 1989[25]. The reaction mixture (3 ml) comprised of 1 ml FeSO4 (1.5 mM), 0.7 ml hydrogen peroxide (6 mM), 0.3 ml sodium salicylate (20 mM) and varying concentrations of the leaf extracts (0.5-3.5 mg/ml). The detection of the resultant hydroxylated salicylate complex was made at 562 nm after incubating the reaction mixture at 37ºC for 1h. Ascorbic acid was used as the standard. |
| J. Statistical analysis A statistical analysis was made using the student t-test to determine the level of significance of the values [26].The values in the tables are expressed as mean ± SD (standard deviation). The p value of less than 0.05 is taken as significant. |
III RESULTS AND DISCUSSION |
| A. Total Phenolic Content (TPC) Total phenolic content was measured according to Folin - Ciocalteu method. The results showed that all the leaf samples used for the present investigation contain considerable amount of polyphenols. The results are expressed as mg equivalents of gallic acid. Further, the results as depicted in Figure1 show that that the dried leaves of C. asiatica have the highest TPC of 2.69%±0.198 followed by M. oleifera and B. alba with a TPC of 2.23%±0.176 and 1.22%±0.039 respectively. TPC values obtained in this study are in agreement with the results published recently with obtained values ranging from 3.23–11.7 g/100 g for different parts of C. asiatica[27], 2.20% TPC for M. oleifera[28] and the results obtained for B. alba are in agreement with the results obtained [29]. |
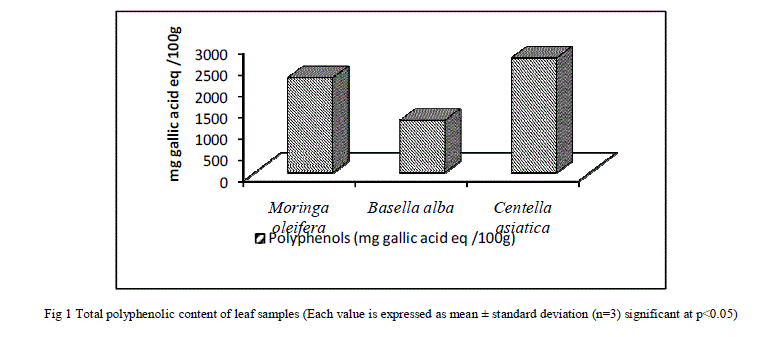 |
| B. Total flavonoid content Total flavonoid contents were successfully analyzed in the leaf samples and expressed as catechin equivalents. Results presented in Figure 2 show that C. asiatica demonstrated the highest flavonoids content of 0.741%±0.0548 followed by M. oleifera and B. alba with a flavonoids content of 0.441%±0.0546 and 0.0952%±0.039 respectively (Figure2). The above results are in comparison to values obtained by Pittella et al (2009) [15], who obtained a total of 0.361% flavonoids in C. asiatica. B. alba depicted a flavonoids content of 10.23mg/g which is in comparison with the results obtained in the present study[10] |
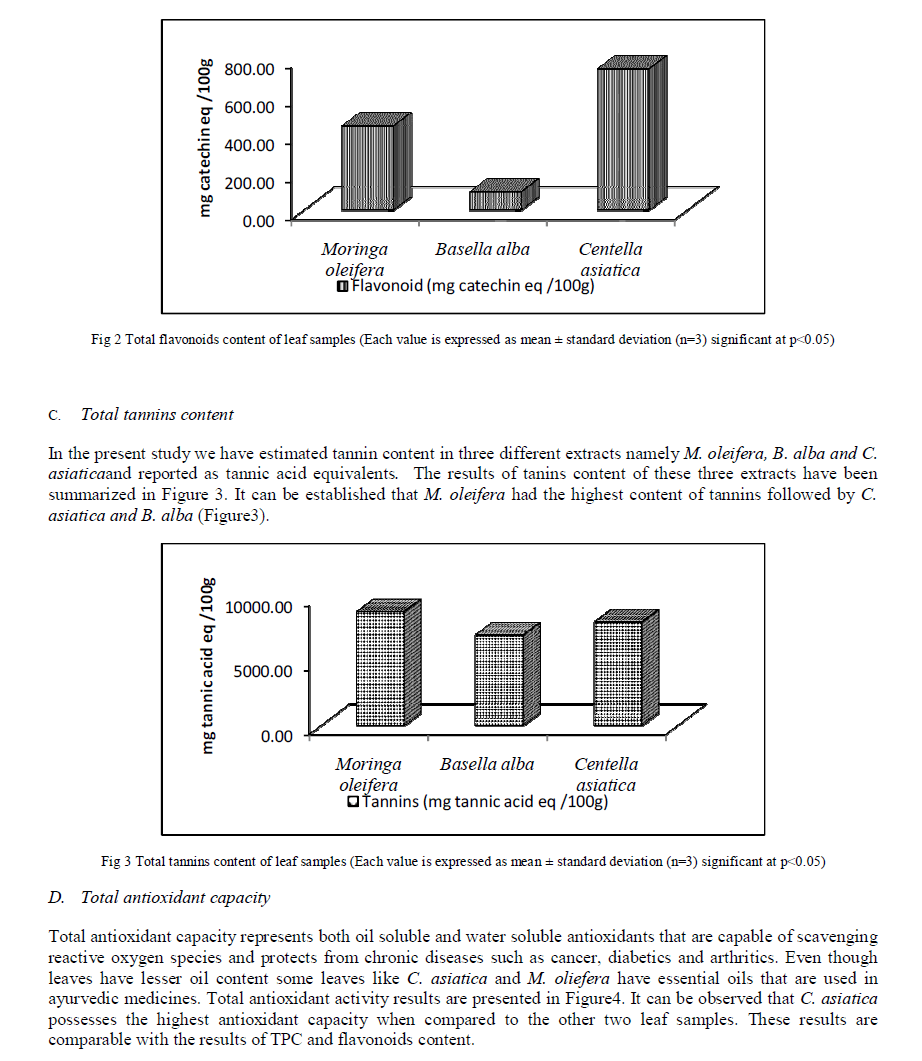 |
 |
 |
IV DISCUSSION |
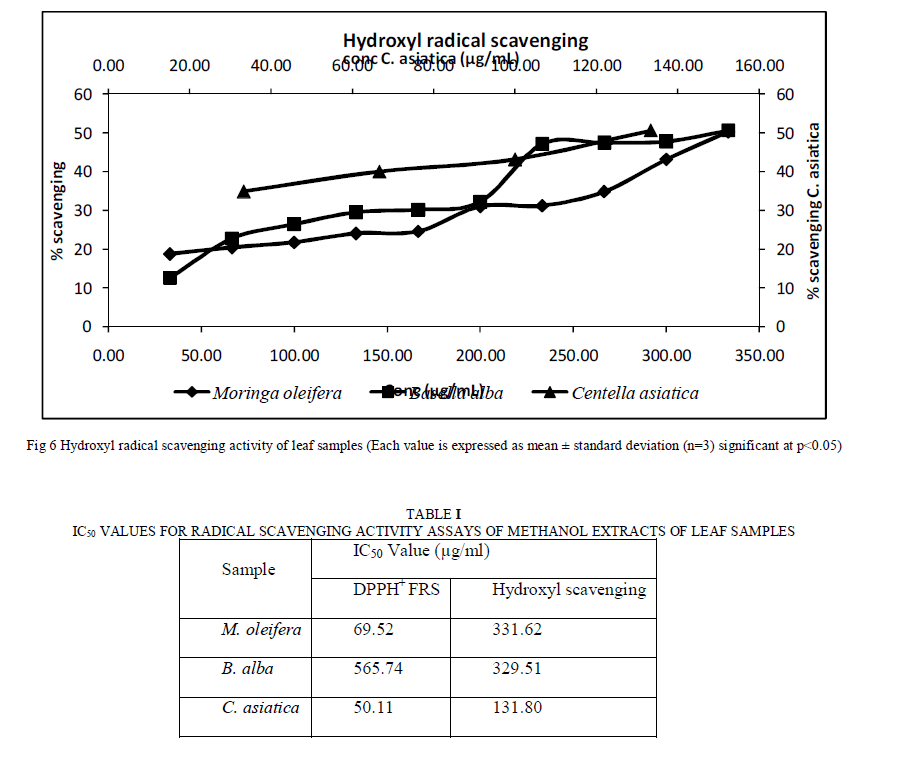
| The results as depicted in Figure1 show that that the dried leaves of C. asiatica have the highest TPC followed by M. oleifera and B. alba respectively. These results can be correlated with the results of total antioxidant activity and free radical scavenging activity assay which is discussed in the later sections. TPC values obtained in this study are in agreement with the results published recently with obtained values ranging from 3.23–11.7 g/100 g for different parts of C. asiatica[27], 2.20% TPC for M. oleifera[28] and the results obtained for B. alba are in agreement with the results obtained by Maisuthisakul et al [29]. At these concentrations, simple phenols do not produce any adverse effects when consumed by animals [31]. However, these phenols have been reported to have multiple beneficial biological effects [32]. A recent report [33] showed that the method used quantifies mainly high-molecular weight phenolic, such as hydrolysable tannin and other polyphenols that have a molecule of gallic acid in its structure, absorbing energy at the wavelength of 760 nm. Therefore gallic acid was chosen as a standard for the present study. The Folin–Ciocalteu method is the one adopted in almost all the published works about screening of natural antioxidants, being considered the best method for determination of total phenolic content. It is well known that Folin–Ciocalteu’s phenol reagent gives different responses to different phenolic compounds, depending on chemical structure [34]. Plants contain various classes of phenolic compounds. Even though a variety of plants are known to be sources of phenolic compounds, data on their composition are insufficient. Plant flavonoids, in general, are highly effective free-radical scavengers and antioxidants. Total flavonoid contents were successfully analyzed in the leaf samples and expressed as catechin equivalents. Results presented in Figure2 show that C. asiatica demonstrated the highest flavonoids content followed by M. oleifera and B. alba. Flavonoids act as antioxidants and this is depicted in the later sections of the study. The values obtained in the present investigation are not matching exactly with the values obtained in previously published works. This may be due to varying experimental conditions and different sources of obtaining the leaf. The results of tannins are contradicting with results of TPC (Figure1) and total flavonoid content (Figure2). In general higher value of tannin corresponds to higher TPC value. We however have found a reverse trend. This might be due to variation in the habitats which in turn has a role to play in the composition of phytochemicals in different parts of the plant [35]. Even though hydrolysable tannin content is found to be high; it is presumed to be safe as they possess health beneficial effects such as anti-diabetic and antioxidant activity. The results of Total antioxidant capacity are comparable with the results of TPC and flavonoids content. It can be said that the different antioxidant activities of the leaf samples can be ascribed to their total phenolic concentrations. When comparing data in Figure1 and Figure2 with Figure4, it can be observed that C. asiatica had the highest phenolic (Figure1) and flavonoids (Figure2) content followed by M. oleifera and B. alba. Several comprehensive works have been done on the effects of phenolic compounds on total antioxidants [36] and correlations between phenolic compounds and total antioxidants. This same trend was also obtained in our study. Hence it can be concluded that the antioxidant activity of leaf extracts is as a result of phenolic compounds. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides [19]. DPPH is relatively stable nitrogen centered radical that easily accepts an electron or hydrogen when reacts with suitable reducing agents as results of which the electrons become paired off and the solution losses color depending on the number of electrons taken up [37]. The hydroxyl radical scavenging activity can be measured by studying the affinity of plant extracts for hydroxyl radicals generated with Fe3+. This reaction can be quantified spectrophotometrically. It can be observed that C .asiatica possesses the highest free radical scavenging capacity when compared to the other two leaf samples. These results are comparable with the results of TPC (Figure1) and flavonoids (Figure2) content. Recent studies have shown that many poly phenols contribute significantly to the antioxidant activity and act as highly effective free radical scavengers due to their redox properties that allow them to act as reducing agents [38]. When comparing data of TPC and flavonoids with that of DPPH+ radical scavenging and hydroxyl radical scavenging, it can be said that radical scavenging capacity of each extract might be related to their concentration of phenolic hydroxyl group. Based on previous data, it is possible that the powerful antioxidant activity of extracts is due to the presence of substances with free hydroxyls. In this context, flavonoids possess an ideal structure for the scavenging of free radicals, since they present a number of hydroxyls acting as hydrogen-donators which makes them important antioxidant agents [39]. The presence of reductive Phytonutrients is reflected by radical scavenging activity assays. It can therefore be concluded that radical scavenging activity of different extracts may be the contribution of one or more antioxidant phyto nutrients |
References |
|