ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Anima Upadhyay, M. Chandrakala Assistant Professor, Department of Chemistry, Sir M. Visvesvaraya Institute of Technology, Banglore, Karnataka, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present study was made to assess the water quality of Ulsoor Lake, Bangalore, India before and after the monsoon season. To carry out the study, samples were collected from the lake before and after the commencement of Rains. Various parameters such as pH, Temperature, Electrical Conductivity(EC), Total dissolved solids(TDS), Total hardness(TH), Total alkalinity(TA), concentration of Ca2+ and Mg2+ ions etc were determined. These parameters were then compared with the WHO and ISI standards to draw the final conclusion on the quality of the lake water before and after the rainy season.
Keywords |
| Ulsoor Lake, Hardness, Turbidity, pH, Conductivity |
INTRODUCTION |
| Bangalore city has many lakes most of them were constructed in the 16th century. These were the sources of water for drinking, agriculture and other useful purposes, as there were no rivers close to the city. With increasing urbanization in Bangalore the fate of the lakes suffered a lot due to dumping of the effluents, sewage, debris, erosion of the soil, disposal of the garbage into the lake waters leading to the pollution and degradation of lakes. Ulsoor Lake is one of the biggest lakes in Bangalore and is located in the east of the city. It is spread over 50 ha and have many islands. It was constructed by Kempe Gowda II and in present times it is the only surviving tank built by Gowda kings. Studies of Physico-Chemical parameters on various lakes in India including Bangalore have been reported by many researchers [1-9]. These studies have proved that the water quality of lakes is deteriorating very fast. Hence study of the quality of lake waters on a regular basis has become inevitable before they become a pool of diseases. Present study is a step towards it. |
II. MATERIALS AND METHODS |
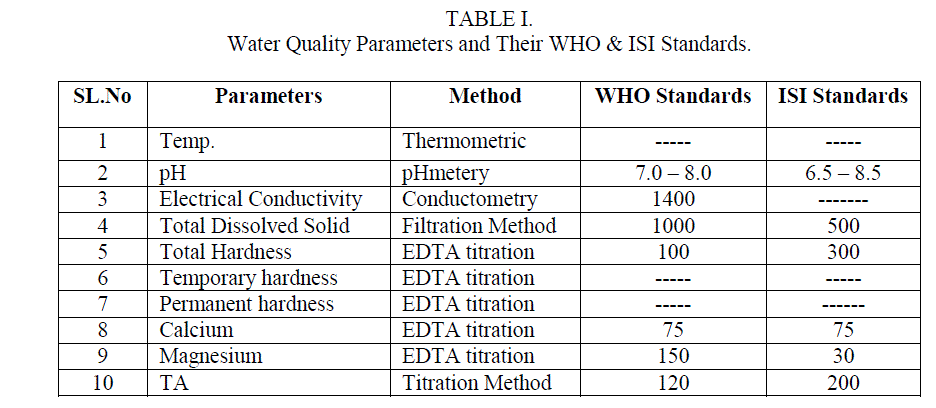
| Samples were collected from Ulsoor lake Bangalore before (S1) and after (S2) the commencement of Monsoon. The Physical and Chemical parameters were studied and the results were compared with the values of WHO and ISI standards[10]. The solutions used for the determination of parameters were prepared from AR grade chemicals, in double distilled water.Water analysis was carried out by standard methods [11]. Electrical Conductivity and pH were determined using Systronics – Conductometer, Digital Systronics and pH – meterrespectively. The Total hardness, Temporary hardness, Permanent hardness, Calcium and magnesium ions were determined by EDTA complexometric titration method. Alkalinity and Total dissolved solids by titration and filtration methods respectively. Temperature of the samples was noted at their sampling points. The methods employed for determination of various parameters and WHO and ISI standards are listed in Table 1. |
III. DETERMINATION OF WATER QUALITY PARAMETERS |
| The parameters studied in the present study were compared with WHO and ISI standards. This formed the basis of results and discussion. The studies involved determination of Total hardness, Temporary hardness, Permanent hardness, Alkalinity, Total dissolvedsolids, concentration of Calcium ions andMagnesium ions. |
IV. RESULTS AND DISCUSSION |
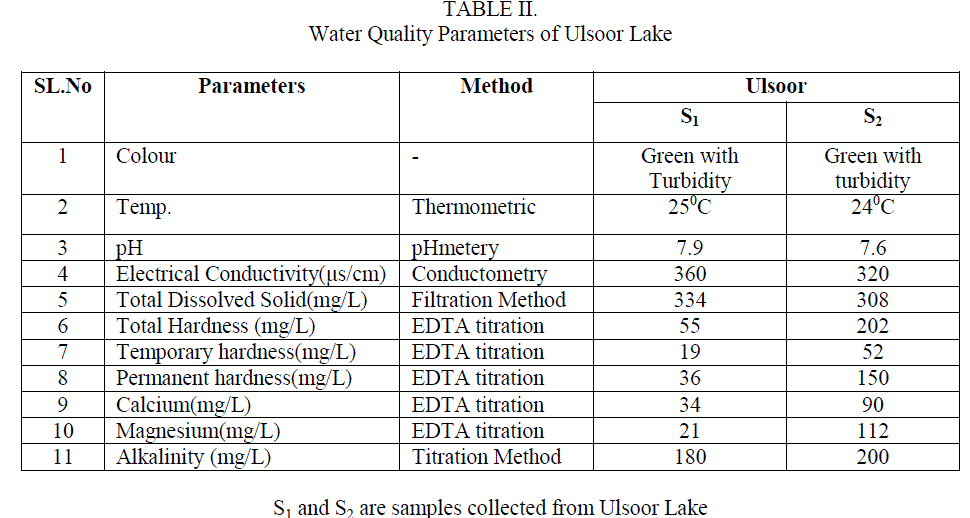
| The water quality was analyzed by collecting samples from Ulsoor Lake. Sample S1 was collected before and S2 after the monsoon. The results obtained by the analysis of these samples are tabulated in table 2 and there comparison with the WHO and ISI standards aretabulated in table 1. |
| 1. Temperature Temperature of water is very important as it speeds up the chemical reactions in water, decreases the solubility of gases,increases the dissolution of salts and amplifies the tastes and odour. During the sampling time the temperature of water was recorded as 250C and 240C for sample S1 and S2 |
| 2. pH pH was found to be 7.9 and 7.6 for S1 and S2respectively. The values are found to be on the higher end according to the WHO standards but when compared to ISI standards it fall well within the limits.Table2 Fig. |
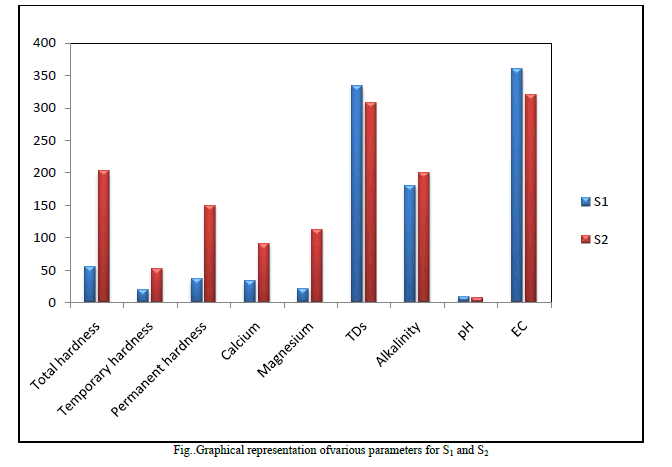
| 3. Electrical Conductivity(EC) Electrical Conductivity is the measure of the waterâÃâ¬ÃŸs ability to conduct electric current. This was found to be 360 μs/cm for S1 and 320 μs/cm for S2 which is not a subject of concern as it was within the limits of both WHO and ISI standards. Table2 Fig. 1 |
| 4. Total Dissolved Solids (TDS) The natural waters contain many dissolved solids mainly chlorides, sulphates, phosphates, nitrates, magnesium, calcium, sodium, potassium, iron, manganese, bicarbonates, carbonates, etc. The dissolved solids are due to the dissolution of rocks, soil minerals, lime, gypsum, etc. The TDS found in the analysis was 334 and 308 mg/L for sample S1 and S2 respectively these values were though within the limit of the said standards but still quite high. Table2 Fig. 1 |
| 5. Total, Temporary and Permanent Hardness Hardness in water is caused due to the presence of dissolved salts of calcium and magnesium ( bicarbonates, chlorides and sulphates), their presence in water leads to many difficulties such as increase in the boiling point of water, wastage of soap while washing clothes or bathing, wastage of fuel when cooking etc. Hence it is an important parameter to be studied S1 and S2 sample gave 55 mg/L and 202 mg/L of total Hardness which according to WHO standard and ISI standards were within the range but sample S2 which was collected after the monsoon showed the values of total hardness to be very high compared to S1 collected before the monsoon. Permanent hardness caused due to the presence of chlorides and sulphate salts of Calcium and Magnesium, was found to be 36 mg/Land 150mg/L for samples S1 and S2 respectively. The bicarbonates of Calcium and Magnesium dissolved in water is the cause for the temporary hardness in the water and this was found to be 19 and 37 mg/L in S1 and S2 respectively. Table2 Fig. 1 |
| 6. Concentration of Calcium and Magnesium ions in sample water Concentration of Ca2+ was found to be 34 mg/L and 90 mg/L samples for S1 and S2 respectively which showed that S2 greatly exceeded the standard values and is a subject of concern whereas Mg2+ was found to be 21 mg/L and 112 mg/L for S1 and S2 samples which was according to WHO standards is within the limit but according to ISI standards S2 was found to be very high. Table2 Fig. 1 |
| 7. Alkalinity Alkalinity of water is described as the capacity of water to neutralize an acid. It is due to the presence of bicarbonates, carbonates and hydroxide ions in water. The alkalinity of the samples S1 and S2 was found to be 180 and 200mg/L respectively which was found to be very high in both the samples. Table2, Fig. 1 |
 |
 |
 |
V. CONCLUSION |
| The present study suggests that the Ulsoor Lake water showed high pH and high Alkalinity but other parameters were within the tolerance limits suggested by the World and ISI standards before the commencement of rains, but after the rains besides these two parameters the other parameters such as hardness, concentration of calcium and magnesium ions also increased drastically making the water highly polluted and unsuitable for use. The cause of this could be the inflow of drainage water into the lake during the monsoon season loaded with dissolved impurities. If measures are taken to control this inflow of water into this lake by constructing walls and proper ducts provided with natural filters like stones and pebbles that lead the water into the lake, it can be preserved and prevented from getting contaminated from the city waste and could result into a wonderful water reservoir for the city that can suffice the purpose of water shortage in the city throughout the year . |
ACKNOWLEDGEMENTS |
| The authors are grateful to the authorities and management of Sir MVIT for their support to conduct this study. |
References |
|