e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Department of Pharmaceutics, Bapatla College of Pharmacy, Bapatla, Guntur Dist - 522101, Andhra Pradesh, India
Received: 13/09/2013 Accepted: 30/12/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Floating microspheres of Ritonavir was prepared by ionic gelation method with an aim of increasing the gastric residence time and for controlled release. Sodium alginate, polymeric mixture of Sodium alginate and Guargum were used as polymers. Sodium bicarbonate was used as the gas-forming gent. The prepared floating microspheres were evaluated with respect to particle size distribution, floating behaviour, drug content, entrapment efficency, morphology and in vitro release study. These results indicated that the release rate was found to decrease with increase in concentration of coating material applied. The wall thickness of microspheres was found to be increased with the increase in concentration of coating material applied. The floating microspheres followed zero order kinetics and the mechanisam of drug release was governed by peppas model. For all the microspheres the exponential coefficient values were found to be in between 0.7664 and 0.8565., indicating non fickian diffusion controlled release mechanism.
Ritonavir, Floating microspheres, Sodium alginate, Sodium bicarbonate, Guargum
Oral controlled release dosage forms (OCRDFS) are being developed for the past three decades due to their advantages. The design of oral controlled drug delivery system is primarily aimed at achieving more predictable and increased bioavailability, thereby obtaining a maximum therapeutic effect. However some of these systems don’t work as planned due to several physiological difficulties, such as an inability to restrain and localize the drug delivery system within desired region of GI tract and highly variable nature of gastric emptying process. It can be anticipated that, depending upon the physiological state of subject and the design of pharmaceutical formulation, the emptying process can last from a few minutes to 12 hours. Rapid GI transit can prevent complete drug release in the absorption zone and reduce the efficacy of administered dose since the majority of drugs are absorbed in stomach or upper part of small intestine [1].
Thus placement of drug delivery system in a specific region of the GI tract offers a numerous advantages especially to the drugs having narrow absorption window, stability problem in intestine, poor solubility in alkaline PH, local activity of in stomach and property to degrade in colon. Therefore the design of a sustained release preparation requires both prolongation of gastrointestinal transit of dosage form as well as controlled drug release. Recently one of such systems has been reported as floating drug dosage systems (FDDS) [2,3]. FDDS have a lower density than gastric fluids and thus remain buoyant in the stomach, without affecting the gastric emptying rate for a prolonged period of time. While the systems are floating, the drug is released slowly from the system at a desired rate. Ritonavir is a antiretroviral agent used in treatment of HIV and viral diseases has been taken as a model drug in the present investigation because of its low biological half-life (3-5h) moreover It is primarily absorbed from stomach [4].
Ritonavir was obtained as a gift sample from Aristo, Bhopal (MP). Sodium bicarbonate, calcium chloride, acetic acid, used was of analytical grade, purchased from Merck specialties Pvt Ltd, Chemistry-chem. Ltd, Loba chemie Pvt Ltd. Mumbai respectively. Guargum was purchased from Yarrow chemical products, Ambala, Sodium alginate was purchased from Sd.Fine Chemicals, Mumbai.
The floating microspheres containing Ritonavir were prepared by orifice ionic gelation technique. Sodium alginate alone or in combination with guargum and the gas forming agent sodium carbonate were dispersed in the purified water to form a homogeneous polymer mixture. The drug, Ritonavir was added to the polymer dispersion and mixed thoroughly on a magnetic stirrer to form a homogeneous dispersion. The gelation medium was prepared by dissolving calcium chloride in 2% glacial acetic acid. The homogenous alginate solution was extruded using 21G syringe needle into the gelation medium. The distance between the edge of the needle and surface of gelation medium was about 10cms. The gel microspheres formed were left in the solution with gentle stirring for 30 min at room temperature to improve mechanic strength. After that, microsphere was collected and washed with distilled water twice, dried at room temperature for 24 hr and stored in desiccators. The composition and the conditions observed during the preparation of microspheres are showed in table no 1.
The flow properties of prepared microspheres were investigated by measuring the bulk density, tapped density, Carr’s index and packing factor. The bulk and tapped densities were measured in a 10 ml graduated measuring cylinder. The sample contained in the measuring cylinder was tapped mechanically by means of constant velocity rotating cam. The initial bulk volume and final tapped volume were noted from which, their respective densities were calculated.
Microspheres were separated into different size fractions by sieving for 10 minutes using a mechanical shaker (Labtech, Indore, Co. India) containing standard sieves # 16, # 24, # 30, # 44 and # 60 and mean particle sizes of microspheres were calculated.
In vitro evaluation of floating behaviour studies were performed by placing 50 particles into 50 ml glass flask and subsequent addition of 50 ml 0.1 N HCl containing 0.02% w/v. Tween 20 was added to exclude floating due to non wetted surfaces followed by horizontal shaking (37 0, 75 rpm). At pre determined time intervals (2, 4, 6, 8 hrs) the flasks were allowed to stand to 5 mins without agitation and numbers of floating particles were counted. The % of floating microspheres was calculated by following equation.
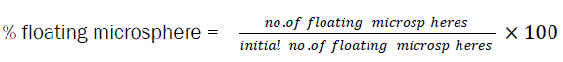
50 mg of formulations was dissolved in 50 ml of 0.1N HCl. The samples were assayed for drug content by UV- spectrophotometer (UV-1700) at 284 nm and the drug content was calculated.
The drug release rates from floating microspheres were carried out using Tablet dissolution test apparatus. A weight of floating microspheres corresponding to 100 mg of drug was filled into a capsule and placed in basket. Dissolution media was 500 ml 0.1N HCl maintained at 37 ± 10 and stirred at 100 rpm. Samples (5 ml) were withdrawn at suitable interval of time and volume was adjusted. It was then assayed spectrophotometrically at 284 nm.
Drug-polymer interactions were studied by FTIR spectroscopy. The spectra were recorded for pure drug, pure polymer and drug-loaded microspheres using FTIR. Samples were prepared in KBr desks [2mg sample in 200mg KBr]. The scanning range was 400-4000cm-1 and the resolution was 2cm-1.
Floating microspheres of Ritonavir were prepared by ionic gelation technique. This process produced uniform microspheres. Microspheres were developed with 1:1, 1:2, 1:3 ratios of core:coat to determine the affect of coating material concentration on the release rate of Ritonovir. These microspheres were characterized for flow properties and the results are given in Table 2. All the formulations offered good flow property. The microspheres also evaluated for size analysis, Drug Content and % Encapsulation Efficiency. The results are given in Table 3. The technique also showed good entrapment efficiency. The microspheres were subjected to In-vitro release studies by employing 0.1N Hydro chloric acid and the data was shown in Figure 1&2.When the amount of drug release values were plotted against time straight lines were obtained in all the cases indicating that the rate of drug release from these microspheres followed zero order kinetics (Fig 3&4) . To ascertain the mechanism of drug release from various microspheres, plot of log %Released vs log time (peppas plots) were drawn. The plots were found to be linear (Fig 5&6). For all the microspheres the exponential coefficient values were found to be in between 0.7664 and 0.8565., indicating non fickian diffusion controlled release mechanism. These results indicated that the release rate was found to be decrease with increase in concentration of coating material applied. The wall thickness of microspheres was found to be increased with the increase in concentration of coating material applied. There exists a good correlation ship in between wall thickness and release rate constant. Drug polymer interactions were studied by FT-IR analysis. Figure: 7 showed the IR spectra of pure Ritonavir . The characteristic CH stretching, NH stretching of secondary amine, C=C stretching and C=O stretching of pure drug was observed at 2964.47 cm-1, 3357.73 cm-1, 3025.35cm-1 and 1714.67 cm-1. The characteristic peaks confirmed the structure of Ritonavir. The same peaks were also reported in all drug loaded microspheres. There was no change or shifting of characteristic peaks in drug loaded microspheres suggested that there was no significant drug polymer interaction which indicates the stable nature of drug in all formulations.
Floating microspheres of Ritonavir prepared by ionic gelation technique were found to be suitable for controlled release. The floating microspheres prepared with sodium alginate and guargum in 1: 3 ratio show prolonged release rate when compared with other formulations.