e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
1College of Pharmacy, Shree Venkateswara College of Paramedical Sciences, Erode-Gobi Main Road, Erode, Tamil Nadu, India
2Mother Theresa Post Graduate and Research Institute of Health Sciences, Puducherry, India
Received date: 09/02/2021; Accepted date: 26/02/2021 ; Published date: 05/03/2021
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Objective: The aim of the study is the preparation of low molecular chitosan using enzymolysis technique. Materials and methods: The high molecular weight chitosan was reduced to low molecular weight using cellulase and pectinase. The prepared low molecular weight chitosan was then evaluated for its viscosity average molecular weight (using Ostwald viscometer), physicochemical parameters and Fourier transforms infrared spectrometer (FT-IR) analysis. Results: It was found that cellulase and pectinase in combination effectively degrades chitosan to produce low molecular weight chitosan and there was no significant difference in the structure rather than molecular weight as shown by FTIR-spectroscopic study. Low molecular weight chitosan having viscosity average molecular weight of 15 KDa was prepared by enzymolysis of cellulase and pectinase under 5 hrs of reaction time. Conclusion: Enzymolysis is the effective and safe method in the degradation of the molecular weight of polymers like chitosan.
Enzymolysis; Cellulase; Molecularchitosan; Viscosity
Chitosan is commercially produced by the deacetylation of chitin, composed of β-(1-4) linked 2-amino -2-deoxy –D-glucopyranose and 2-acetamido-2-deoxy-D-glucopyranose. Chitosan have received much attention for a wide range of unique applications in food, pharmaceutical, cosmetics, agricultural and medicinal fields. Chitosan is well soluble in diluted acids, its solutions, however even at low concentrations (0.1%-0.3%) are water insoluble and are characterized by high viscosity and fishy bitter astringent taste, which limits their uses in practice. Low molecular weight Chitosan (LMWC) has become a topic of growing interest. Low molecular weight Chitosan have higher solubility in neutral aqueous solutions than high molecular weight chitosan, which broadens their application as e.g. antimicrobial, antifungal and antitumor agents [1-4].
Low molecular weight Chitosan (LMWC) can be prepared by chemical or enzymatic depolymerisation of Chitosan. The chemical approach can be performed by acidic or oxidative depolymerisation. The acidic depolymerisation is carried out using high concentration of hydrochloric, phosphoric or sulphuric acids. Enzymatic methods are based either by specific enzymes, such as chitosanases [5-7] or by using nonspecific enzymes [8,9] including lysozymes, cellulases, lipases, amlyases, papain and pectinases [10-15].
Drawbacks of physical and chemical method
Chemical degradation however some defects, including harsh hydrolysis conditions, low yields chemical modification the glucose rings. Physical degradation of chitosan requires special equipment, and the resulting molecular weight cannot be controlled [16-17].
Advantages of low molecular weight chitosan
• Low molecular weight Chitosan has higher solubility in neutral aqueous solutions than high molecular weight Chitosan.
• High molecular weight Chitosan is characterized by fishy bitter astringent taste, which limits its usage. Water Soluble Low molecular weight Chitosan has no bitter taste.
• Low molecular weight Chitosan with average molecular weight of 5-20 K Da has enhanced functional-biochemical significance. 5-10 K Da showed stronger growth inhibitory effect on several pathogens including Fusarium oxyporum, Phomopsis fukushi, Alternaria alternate [18].
• Average molecular weight more than 5 KDa prevented the serum rise of cholesterol of rats fed cholesterol enriched diets for 14 days
• Low molecular weight chitosan also reduced the incidence of early pre-neoplastic markers of colon carcinogenesis [18-23].
• Chito-hexamer suppressed Sacroma -180 and Meth-A tumor growth in mice [18-22]. Low molecular weight chitosans are used as antitumor and immune-stimulating agents.
The materials used in the preparation of low molecular weight chitosan are chitosan (HIMEDIA) Acetic acid (RANKEM), cellulase and pectinase Gift sample (sun glow pharmaceuticals) and Sodium chloride (Chemspure).
Preparation of low molecular weight chitosan by enzymolysis
Chitosan (1.0%W/V) solution was prepared using 1%v/v acetic acid, which having a pH of around 3.0. To the above solution enzymes was added and incubated at 37°C for 3-5 hrs with constant stirring [19,20]. After the hydrolysis, the enzyme was inactivated at 1000°C for 10 min and the pH was adjusted to 12 with 2 M NaOH to precipitate the products with a high degree of polymerization (DP). The suspension was centrifuged; the insoluble residue was washed with double distilled water and lyophilized to give LMWC (Table 1).
| Sample | Cellulase | Pectinase | Time of degradation (hrs) |
|---|---|---|---|
| Chitosan (1%w/v) | 0.3 g | - | 3 |
| Chitosan (1%w/v) | - | 0.3 g | 3 |
| Chitosan (1%w/v) | 0.3 g | 0.3 g | 3 |
| Chitosan (1%w/v) | 0.3 g | - | 4 |
| Chitosan (1%w/v) | - | 0.3 g | 4 |
| Chitosan (1%w/v) | 0.3 g | 0.3 g | 4 |
| Chitosan (1%w/v) | 0.3 g | - | 5 |
| Chitosan (1%w/v) | - | 0.3 g | 5 |
| Chitosan (1%w/v) | 0.3 g | 0.3 g | 5 |
Table 1: Preparation of low molecular weight chitosan by enzymolysis.
Evaluation of prepared low molecular weight chitosan
Determination of average viscosity molecular weight by Ostwald’s viscometer: The viscosity average molecular weight was determined by using Ostwald viscometer. Solvent used in this work was a mixture of 10 ml of 0.5 M acetic acid and 20 mlof 0.25 M sodium chloride [19]. The experiment was carried out in triplicate to get concordant value. The average viscosity molecular weight was then calculated from the following formulas.
Relative viscosity
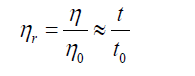
Relative viscosity increment (or specific viscosity) is the ratio of difference in viscosities (or efflux times) to solvent viscosity (or solvent efflux time)
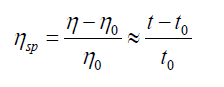
Reduced viscosity (or viscosity number)
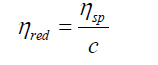
Inherent viscosity
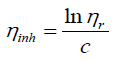
Intrinsic viscosity [η] can be defined:
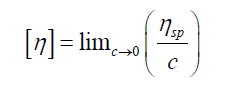
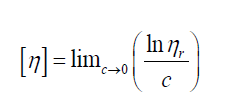
By plotting viscosities as a function of concentration the intrinsic viscosity can be estimated by extrapolation of polymer solution to zero concentration.
Mark-Houwink equation: The equation describing the dependence of the intrinsic viscosity of a polymer on its relative molecular mass (molecular weight) is:
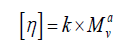
Where [η] is the intrinsic viscosity, K and a are constants the values of which depend on the nature of the polymer and solvent as well as on temperature, and M is usually one of the relative molecular mass averages.
Appearance: The appearance of the prepared low molecular weight chitosan was studied.
Tapped density: The prepared low molecular weight chitosan and raw chitosan was weighed, collected, and poured into a 10 ml of graduated cylinder. This system was tapped for 100 times from 2.5 cm height and then the volume of filled chitosan and low molecular weight chitosan was measured. Tapped density was calculated by using the following formula:
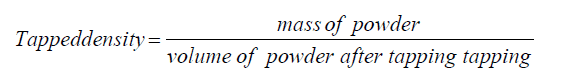
Bulk density: Apparent bulk density was determined by placing chitosan and low molecular weight chitosan into a graduated cylinder and the volume and weight was measured as it is.
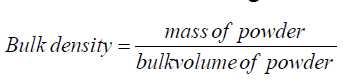
Percentage compressibility index: Compressibility is the ability of powder to decrease in volume under pressure. It is one of the methods to determine the flow properties by comparing the bulk and tapped density (Table 2).
| Percentage compressibility | Flow description |
|---|---|
| <10 | Excellent |
| 11-15 | Good |
| 16-20 | Fair |
| 21-25 | Passable |
| 26-31 | Poor |
| 32-38 | Very poor |
| >40 | Extremely poor |
Table 2: Percentage compressibility index.
The prepared low molecular weight chitosan and chitosan was weighed, collected, and poured in to a 10 ml of graduated cylinder. This system was tapped 100 times and then the volume of filled chitosan was measured. It is the ratio of the volume before tapping which was filled in the graduated cylinder and after tapped volume.

Where V and Vo are the volume of the samples after tapping and before tapping.
Fourier transforms infrared spectrometer (FT-IR) analysis: FTIR spectroscopy analysis of low molecular weight chitosan was done using the KBr disc technique in the range of 4000-400 cm—1. All the spectra were recorded at room temperature with a resolution of 4 cm—1 for 45 scans [21-23]. FTIR study was done to confirm that there is no structure variation in the resulting low molecular weight chitosan when compared to normal chitosan.
Evaluation of prepared low molecular weight chitosan
Determination of viscosity average molecular weight of chitosan: The viscosity average molecular weight of chitosan and prepared low molecular weight chitosan products of various time intervals was calculated and the results were described in the Tables 3-5 and Figures 1-2.
| Sample | Concentration (g/ml) | Time (secs) | Relative viscosity ηr | Specific viscosity ηsp | Reduced viscosity, ηsp / concentration | Inherent viscosity |
|---|---|---|---|---|---|---|
| C0 | 0 | 31 | 1 | 0 | 0 | 0 |
| C1 | 0.0006 | 69.74 | 2.24968 | 1.24968 | 2082.8 | 1351.31 |
| C2 | 0.001 | 76.99 | 2.48355 | 1.48355 | 1483.55 | 909.688 |
| C3 | 0.002 | 85.1 | 2.74516 | 1.74516 | 872.581 | 504.92 |
| C4 | 0.0026 | 86.67 | 2.79581 | 1.79581 | 690.695 | 395.431 |
| C5 | 0.0033 | 93.33 | 3.01065 | 2.01065 | 609.286 | 333.986 |
Table 3: Evaluation of chitosan-average molecular weight by viscosity.
| Sample | Concentration (g/ml) | Time (sec) | Relative viscosity ηr | Specific viscosity ηsp | Reduced viscosity ηsp / conc | inherent viscosity |
|---|---|---|---|---|---|---|
| C0 | 0 | 31 | 1 | 0 | 0 | 0 |
| C1 | 0.0006 | 31.56 | 1.01806 | 0.01806 | 30.1075 | 29.8388 |
| C2 | 0.001 | 31.57 | 1.01839 | 0.01839 | 18.3871 | 18.2201 |
| C3 | 0.002 | 31.91 | 1.02935 | 0.02935 | 14.6774 | 14.4661 |
| C4 | 0.0026 | 31.95 | 1.03065 | 0.03065 | 11.7866 | 11.6096 |
| C5 | 0.0033 | 32.57 | 1.05065 | 0.05065 | 15.347 | 14.971 |
Table 4: Evaluation of prepared low molecular weight chitosan by cellulase and pectinase-5 hrs.
| S.no | Time of degradation (hrs) |
Initial molecular weight of chitosan (Da) |
Final Molecular weight of chitosan Using cellulase (Da) |
Final Molecular weight of chitosan Using pectinase (Da) |
Final Molecular weight of chitosan Using cellulase and pectinase (Da) |
|---|---|---|---|---|---|
| 1 | 3 | 1123197 | 61895 | 57773 | 50207.7 |
| 2 | 4 | 1123197 | 46987.3 | 37869 | 29175.1 |
| 3 | 5 | 1123197 | 20639.4 | 19401 | 14661.8 |
Table 5: Summary of viscosity average molecular weight of LMWC preparation
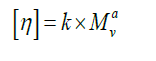
K=0.001424, a=0.96, η= (1115.8+716.42)/2=916
Viscosity average molecular weight, Mv = 1123197 Da
From the Table 5 it was clear that increase in the time of reaction with combined enzymes of cellulase and pectinase significantly decreases the molecular weight of chitosan. The molecular weight of raw chitosan was significantly decreased from 1123197 Da to 15 K Da in the 5 hrs of reaction time. This obtained low molecular weight chitosan product was selected for further evaluation studies.
Appearance: The prepared low molecular weight chitosan were off white colored, moderately fine powder.
Tapped density: The tapped density of chitosan and prepared low molecular weight chitosan was measured and the results are described in the Table 6.
| Chitosan | Bulk density | Tapped density | Carr’s index |
|---|---|---|---|
| High molecular weight | 0.244 g/ml | 0.4 g/ml | 39 |
| Low molecular weight | 0.345 g/ml | 0.53 g/ml | 34 |
Table 6: Comparison of high and low molecular weight chitosan
Bulk density: The bulk density of chitosan and prepared low molecular weight chitosan was measured and the results are described in the Table 6.
Percentage compressibility index: The compressibility index of chitosan and prepared low molecular weight chitosan was measured and the results were described in the Table 6.
The Table 6 given below illustrates the results of the bulk density, tapped density and Carr’s index. From these values, the difference in the density and Carr’s index of low molecular weight chitosan and high molecular weight chitosan are noted. It was evident from the Carr’s index result that both high molecular weight and low molecular weight chitosan have poor flow property.
Fourier transforms infrared spectrometer (FT-IR) analysis: The FTIR spectra study of the prepared low molecular weight chitosan was carried out and compared with the initial chitosan. These results demonstrated that the structures of the main chain of the initial chitosan and LMWCs were the same. The NH2 amino groups had a characteristic peak near 3440 cm-1, which was overlapping by the peak due to the –OH group. The occurrence of absorption peaks at around 2900 cm-1 was assigned to the asymmetric stretching vibration of the –CH2 and the rapid reduction in the intensity for the LMWCs was probably attributed to degradation of chitosan after hydrolysis. A signi?cant peak around 1660 cm-1, which suggests that the –C=O groups had more opportunity to form stronger hydrogen bonds, and the scission of polymer chains led to the decrease of the chitosan molecular weight. The results indicated that there was no signi?cant difference between the main structures of the two samples before and after the enzymatic hydrolysis, but the molecular weight of the main hydrolysis products decreased (Figure 3).
The molecular weight of chitosan was significantly reduced from 1123197 Da to 15000 Da by enzymolysis method using cellulase and pectinase enzymes under 5 hrs of reaction time. The FTIR studies show that there were no significant changes in the structure other than the molecular weight. The physicochemical properties of initial high molecular weight chitosan and low molecular weight chitosan like tap density, bulk density and compressibility index was carried out and the results were compared. It was found that the combined enzymes of cellulase and pectinase are effective in molecular weight reduction, rather than the individual enzymes.
Enzymatic methods of molecular weight reduction are safer and effective than the physical and chemical method of depolymerisation. Enzymolysis by cellulase and pectinase can be used in the weight reduction of polymers like chitosan.