e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Bassam Abualsoud*
Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Al-Ahliyya Amman University, Al-Salt Amman, Jordan
Received date: 24/09/2021; Accepted date: 08/10/2021; Published date: 15/10/2021
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
In this work, Acacia senegal (As) gum was cross-linked by using glutaraldehyde as a cross-linking agent to formulate microspheres. Acacia senegal gum is used traditionally as an excipient in different conventional pharmaceutical dosage forms. This is the first time that this gum is prepared as a cross-linked microsphere. Acacia senegal gum is characterized by safety, biocompatibility, biodegradability and widely availability. Polymer cross-linking reduces the number of hydroxyl groups on the polysaccharide chain, bonding the chains together, hence decreasing its affinity to water and converting the highly soluble gum to water-swellable microspheres that can be used as drug delivery system. In this research Acacia senegal gum microspheres were prepared by emulsification method to prepare water in oil emulsion followed by cross-linking. Characterization of the resultant microspheres was done by using FTIR, DSC, Inverted light microscope, Laser diffraction particle size analyzer, degree of swelling and micrometric properties. The results show that the resultant microspheres percentage yield was in the range of (76.25% to 90.06%), microspheres size (µ) range was between 15.37 µ and 24.14 µ, and degree of swelling was found to be between 3.58 and 4.29. The combined effect of the selected variables on yield, particle size, and the degree of swelling indicated that concentration of Acacia senegal gum, amount of cross-linker (glutaraldehyde) and stirring speed significantly affected the formulation of microspheres. They were the most critical factors in the formulation of microspheres.
Gum arabic; Acacia senegal; Colon targeting.
Natural polymers are synthesized by different living organisms such as animals, plants, microorganisms, and algae. They characterized by biodegradability, biocompatibility, safety, and availability [1]. Plant gums are biopolymeric materials, composed of complex heteropolysaccharides, and proteinaceous material, in addition to some mineral elements [2,3]. Natural gums in general are promising biodegradable polymeric materials and have many advantages over synthetic polymers [4]. The advances in drug delivery technology make natural polymers take an important role in novel drug delivery systems, to fulfil multitask functions, so in some cases directly or indirectly, they control the extent, and rate of drug release [4,5]. However, there is a need to develop new natural improved polymers, by modifying existing natural materials to develop novel drug-delivery systems. Natural gums are metabolized by the normal flora of the intestine and degraded to their component sugars. Enzymes secreted by intestinal microflora can cleave the gums at specific sites leading to gum degradation [6,7].
Carrier technology is an approach for drug delivery by the combination of the drug to a carrier particle such as microspheres, liposome, etc. that regulates the release characteristics of the drug at a fixed rate for a prolonged period. The targeted and controlled drug delivery systems offer many advantages over conventional dosage forms, which include improvement of efficacy, reduction in toxicity, improvement of patient compliance, and convenience [8]. Microspheres are a drug delivery system which are used to obtain prolonged or controlled drug delivery to improve bioavailability, stability and to target the drug to a specific site at a predetermined rate [9]. Microspheres are characteristically free-flowing powders having a particle size ranging from 1 μ-1000 μ [10]. These delivery systems offer numerous advantages compared to conventional dosage forms, which include improved efficacy, reduced toxicity, improved patient compliance and convenience [11]. Microspheres are various types like; bioadhesive microspheres, magnetic microspheres, floating microspheres, radioactive microspheres, polymeric microspheres, biodegradable polymeric microspheres, and synthetic polymeric microspheres are prepared by different methods [12].
A crosslink can be defined as a bond that links the functional groups of a polymer chain to that of another one through different types of bonds (chemical or physical) and forms a three-dimensional network. Cross-linkers such as glutaraldehyde, epichlorohydrin, etc. are widely used in the preparation of the crosslinked hydrogel network of different synthetic and natural polymers. The technique mainly involves the introduction of a cross-linking agent between the polymeric chains to produce cross-linked chains. The cross-linking of natural and synthetic polymers can be achieved through the reaction of their functional groups (such as hydroxyl groups, carboxylic acid groups, and amino groups) with cross-linkers such as aldehyde (e.g., glutaraldehyde, adipic acid dihydrazide) Generally, cross-linking agents are double-headed reagents that interact with functional groups of polymers. Cross-linking of polymers has been widely used in various formulations by different researchers [13]. Glutaraldehyde reacts with the functional groups of proteins and polymers like amine or hydroxyl groups, through a Schiff-base reaction and make a connection via intra- or intermolecular interactions between the biopolymeric chains [14]. Crosslinked As gum microspheres can be a potential carrier for colon-specific drug delivery due to the prevention of drug release in upper parts of the gastrointestinal system because of reduction of gum hydrophilicity by cross-linking.
Gum Arabic is one of the oldest, and best-known among all-natural gums. The usage of Arabic gum dates to 5000 years ago [15]. FAO/WHO (Food and Agriculture Organization/World Health Organisation)Joint Expert Committee on Food Additives (JECFA), in FNP 53 Add 7 (1999) define gum Arabic as “a dried exudate obtained from the stems and branches of Acacia senegal (L.) Willdenow or Acacia seyal (fam. Leguminosae)”. Gum Arabic: Acacia senegal (known as gum hashab), and Acacia seyal (known as gum talha), and both grow across the Sahelian belt of Africa, principally Sudan. Acacia senegal gum is produced from the stems, and branches of Acacia senegaltree (4-15 years old) by a biosynthesis process (gummosis), when subjected to stress conditions. Chemically Acacia senegal consists of a mixture of high molecular weight polysaccharide of 1, 3-linked β-D-galactopyranosyl units (major component), and hydroxyproline-rich glycoprotein (minor component). The basic structural units of the gum are L-arabinose (45%-65%), D-galactose (23%-36%), L-rhamnose (2%-3%), and uronic acid (8%-14%) [16]. In the “gum trade”, the gum obtained from Acacia senegal (L.) Willde now has the greatest commercial value and is recognized as the best in quality. Primarily because of this, over the past fifty or so years, most research concerned with acacia plant gum exudates has focused on the gum obtained from this species. Gum Arabic has been extensively used in its pure form as an emulsifier and thickener; in addition, its modified forms are also reported to be used as hydrocolloids in a variety of applications [6]. It can also be modified.
Mechanically powder Gum Arabic was obtained from the Gum Arabic Company Ltd (Sudan). Glutaraldehyde was purchased from General Drug House (P) Ltd., (INDIA). Span 80 and castor oil was purchased from E Merck, (Darmstadt, Germany). All other chemicals used in the study were of analytical grade.
Method of preparation of cross-linked Acacia senegal gum microsphere
As gum microspheres were prepared by single emulsion method with slight modification [17]. Chemical cross-linking for preparation of As gum microspheres was done by emulsification of 40 ml of different concentrations of an aqueous solution of As gum (an accurately weighed amount of As gum was dispersed in a specified volume of cold water and allowed to hydrate for 2 hours) in 100 ml castor oil containing 3% span 80. The resultant biphasic system was stirred with a mechanical stirrer, at 4000 rpm for 15 minutes. After complete mixing, the pH of the emulsion was adjusted to 3 by using hydrochloric acid. Cross-linking was done by addition of different amounts of 25% Glutaraldehyde, followed by stirring at a constant speed for 5 hours at different temperatures. Before application of the experiments, several preliminary trials were conducted to determine the conditions at which the process resulted in microspheres. Formation and properties of the microspheres such as yield, particle size, and degree of swelling were affected by six different factors: Acacia senegal gum concentration (A), Glutaraldehyde volume (B), Span 80 concentration (C), stirring speed (D), cross-linking time (E), and Temperature of the system (F). To clearly investigate the influence of the six variable factors and maximize the responses; Plackett–Burman design had been applied using Design Expert 8 (DX8) software.
Experimental design
Screening of critical factors influencing Acacia senegal gum microspheres using Plackett and Burman design: The Plackett and Burman design were applied as the primary step in the study to screen the significant factors, which have a considerable effect on the formation and properties of Acacia senegal gum microspheres. Plackett and Burman Design cannot give any significant information regarding the interaction effects amongst all the variable factors. However, it is mainly applied to screen out the important independent variables, which have a major impact on responses (Y). Based on the design, each variable was examined at two different levels: (-1) for low levels and (+1) for high levels and the (-) and (+) values of all six variables have mentioned (Table 1).
| Symbol code | Factors | Experimental values | |
|---|---|---|---|
| A low value (-1) | High value (1) | ||
| A | Acacia senegal gum concentration (%) | 10 | 20 |
| B | Glutaraldehyde volume (ml) | 1 | 3 |
| C | Span 80 concentration (%) | 1 | 3 |
| D | Stirring speed (rpm) | 2000 | 4000 |
| E | Cross-linking time (hrs) | 5 | 7 |
| F | Temperature (oC) | 60 | 80 |
Table 1. Variables and their levels in Plackett–Burman screening design of experiments for Acacia senegal gum microspheres.
The parameter level ranges were selected based on preliminary experiments.
Design-Expert® software (version 8, Stat-Ease) was used to apply Plackett–Burman design with 12 runs, as shown in Table 2. The percentage yield, particle size, and degree of swelling were taken as a dependent variable, and the statistics were applied to find the most critical factors affecting the formulation ofAcacia senegal gum microspheres. The regression analysis result of (p<0.05) significance at the 95% level was considered significant.
| Formulation | A Acacia senegal gum Conc. (%) | B Cross-Linker amount (ml) | C Span 80 conc. (%) | D Stirring speed (RPM) | E Cross-linking Time (hr) | F Temp. (°C) |
|---|---|---|---|---|---|---|
| F1 | -1 | 1 | -1 | 1 | 1 | -1 |
| F2 | -1 | -1 | -1 | 1 | -1 | 1 |
| F3 | 1 | -1 | -1 | -1 | 1 | -1 |
| F4 | -1 | 1 | 1 | -1 | 1 | 1 |
| F5 | 1 | -1 | 1 | 1 | -1 | 1 |
| F6 | -1 | -1 | -1 | -1 | -1 | -1 |
| F7 | -1 | -1 | 1 | -1 | 1 | 1 |
| F8 | 1 | 1 | -1 | 1 | 1 | 1 |
| F9 | 1 | 1 | 1 | -1 | -1 | -1 |
| F10 | 1 | 1 | -1 | -1 | -1 | 1 |
| F11 | 1 | -1 | 1 | 1 | 1 | -1 |
| F12 | -1 | 1 | 1 | 1 | -1 | -1 |
Table 2. Coded values of Plackett–Burman design experimental matrix for Acacia senegal gum microspheres.
Evaluation of Acacia senegal gum microspheres
Fourier transform infrared spectroscopy of Acacia senegal gum microspheres: The spectra were recorded for microspheres using FTIR spectrophotometer (SHIMADZU FTIR spectrophotometer). Samples were prepared by KBr disk method, and scanned over the range of 400-4000 cm-1; the resolution was 2/cm. The spectra were recorded for Acacia senegal gum, and cross-linked gum microspheres to identify certain characteristic bonds on the compounds, that indicate the formation of cross-linked bonds.
Differential scanning calorimetry of Acacia senegal gum microspheres: The thermal behaviour of Acacia senegal gum before, and after cross-linking was examined with DSC1 star system Mettler Toledo, DSC. Approximately 2.5 mg of sample was weighed in an aluminium pan and crimped with aluminium lid by using crimper. The sample was placed in a cell against a reference cell which having empty aluminium pan. DSC curves were recorded at a temperature range of 30°C-450°C. With a heating rate of 50°C per minute.
Shape and surface morphology of Acacia senegal gum microspheres: Surface and shape characteristics of microspheres were evaluated by means of an inverted light microscope (ZEISS Primovert). The samples were analyzed using a magnification of 400 × at room temperature. Photomicrographs of cross-linked Acacia senegal gum microspheres obtained from various batches were taken using a digital trinocular microscope (Axioplan microscope, MPM-400 with image analyzer, Zeiss, Oberkochen, Germany).
Particle size analysis of Acacia senegal gum microspheres: Particle size was determined by using a laser diffraction particle size analyzer (Mastersizer 2000, Malvern, UK). Microspheres were suspended in the chamber of the particle size analyzer containing distilled water, and the particle size was determined using the software provided by the manufacturer.
The percentage yield of Acacia senegal gum microspheres: The percentage yield was calculated as the weight of the microspheres recovered from each batch divided by total weight of polymer used to prepare that batch multiplied by 100. It can be calculated as follow:

Degree of swelling of Acacia senegal gummicrospheres
Effect of microsphere on water absorption capacity was determined by swelling the microspheres in the water at room temperature.
A known weight (50 mg) of microspheres was placed in distilled water and allowed to swell for 24 hrs. The weight of swollen samples was measured after blotting excessive water gently with filter paper. The water absorption (Wsw) of the microspheres was then calculated from the following formula
Wsw = [(W24-W0)/W0]
Where:
Wsw is The percentage of water absorption
W0 is the initial weight of the dry microspheres
W24 is the weight of swollen microspheres at equilibrium swelling.
Micromeritic properties of Acacia senegal gum microspheres
The flow properties of microspheres were investigated by determining the angle of repose, bulk density, and tapped density. The angle of repose was determined by the fixed-base cone method. Bulk and tapped densities were measured in 10 ml of a graduated cylinder. The sample contained in the cylinder was tapped. The tapped volume was noted down when it showed no change in its value, and bulk density and tapped density was calculated. Each experiment was performed 3 times.
Determination of bulk density and tapped density of Acacia senegal gum microspheres
Microspheres (1 gm) were taken into a 10 ml measuring cylinder, and the initial volume was recorded. The measuring cylinder was tapped 50 times using USP bulk density apparatus.
The bulk density and tapped density were determined using the following formula:

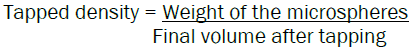
Statistical analysis of data
Data are expressed as mean ± SD. Statistical analysis was carried out using SPSS 18®. Comparison among formulations was made by applying one–way Analysis of Variance (ANOVA). Values of p<0.05 were taken as indicative of statistical significance.
Characterization of microspheres
Absorption band at 3432.21 cm-1 representing the presence of hydrogen-bonded OH group. This peak is broader for Acacia senegal gum than that for cross-linked gum, indicating more OH group than that for cross-linked microspheres. The intense absorption band at 1724.36 cm1 in IR spectra of cross-linked microspheres expresses the carbonyl group which could be due formation of new acetal groups.
This new peak indicates that Glutaraldehyde may react with the hydroxyl of Acacia senegal gum (Figures 1 and 2).
The broader peak at 1244.09 cm-1 and 1072.42 in spectra of cross-linked Acacia senegal gum microspheres as compared to that of Acacia senegal gum indicates ether linkages (cyclic ether large ring–C-O- stretch), reflective of the Glutaraldehyde-cross linked Acacia senegal gum.
The aldehyde groups from Glutaraldehyde reacts with hydroxyl group from polymer under acidic condition and then forms acetal bridges (Figures 3 and 4).
DSC thermal profiles for Acacia senegal gum: an endothermic event at about 80°C. This can be related to the loss of water content in the gum. And another endothermic peak at about 290°C may be due to the melting ofAcacia senegal gum. While an exothermic peak was bobserved at 340°C may be due to polymer decomposition. Meanwhile, microspheres of Acacia senegal gum cross-linked with Glutaraldehyde showed a different pattern of the DSC thermogram with an endothermic peak at 260°C may be due to cross-linked polymer melting and an exothermic peak at 330°C due to cross-linked polymer decomposition reflective of formation of a new compound.
Melting doesn't involve the breaking of covalent bonds. The melting point depends on the intermolecular forces between the chains. Because the crosslink sites are made of polar covalent bonds, the slightly positive or slightly negative charge (i.e. dipole) may attract nearby opposite sites. Such attraction may also contribute to a wider melting range melting temp. Shape and Surface Morphology of Acacia senegal gum shown in Figures 5 and 6.
Figure 5: Trinocular photomicrographs of water in oil emulsion (Acacia senegal gum solution in castor oil).
Note: A graph of Acacia senegal gum solution droplets in castor oil (w/o) emulsion is shown. Graphs of cross-linked Acacia senegal gum microspheres in castor oil before separation are shown at Figure 5.
The combined effect of the selected variables on yield, particle size, and degree of swelling indicated that concentration of Acacia senegal gum, amount of cross-linker (glutaraldehyde) and stirring speed significantly affected the formulation of microspheres. They were the most critical factors in the formulation of microspheres (Figure 7).
Analysis of Variation (ANOVA) helps to identify the significant independent factors that affect the responses and the fitness of the model. It was applied to determine the significance and the magnitude of the effects of the main variable and their interactions by applying probability value (𝑝 value). The fitness of the model was checked by the coefficient of determination (𝑅2) and signal to noise (𝐹-test).
The main factors affecting microspheres percent yield were As gum concentration and amount of Glutaraldehyde. It was observed that the increase in stirring speed decrease the particle size.
Acacia senegal gum concentration had a negative effect on particle size, so particle size increases Acacia senegal gum concentration decrease (Table 3).
| Source | Sum of squares | Degree of freedom | Mean Square | F Value | p -value |
|---|---|---|---|---|---|
| Yield (%) | |||||
| Model | 254.40 | 3 | 84.8 | 75.25 | ˂ 0.0001 |
| Residual | 9.01 | 8 | 1.13 | ||
| Corrected Total | 263.41 | 11 | |||
| Particle size (µ) | |||||
| Model | 90.12 | 3 | 30.04 | 19.99 | 0.0004 |
| Residual | 12.02 | 8 | 1.50 | ||
| Corrected Total | 102.14 | 11 | |||
| Degree of swelling | |||||
| Model | 0.77 | 3 | 0.26 | 45.43 | ˂ 0.0001 |
| Residual | 0.045 | 8 | 0.003 | ||
| Corrected Total | 0.82 | 11 | |||
Table 3. Summary of ANOVA of Plackett and Burman Design response parameters for Acacia senegal gum microspheres.
The summary of the analysis of variance for response parameters is given in Table 4. The F value and p-value suggest that the model is significant for these variables.
| Formulation | Y1 Yield (%) | Y2 Particle size (µ) | Y3 Degree of swelling |
|---|---|---|---|
| F1 | 83.71 ± 3.14 | 15.37 ± 1.17 | 3.65 |
| F2 | 76.25 ± 4.35 | 16.89 ± 1.34 | 4.06 |
| F3 | 81.73 ± 3.89 | 23.42 ± 1.39 | 4.19 |
| F4 | 86.97 ± 4.16 | 19.75 ± 1.31 | 3.58 |
| F5 | 81.49 ± 3.87 | 16.57 ± 1.23 | 4.29 |
| F6 | 77.12 ± 2.54 | 24.14 ± 1.44 | 3.88 |
| F7 | 78.54 ± 3.59 | 19.74 ± 1.10 | 3.81 |
| F8 | 89.15 ± 3.66 | 17.26 ± 1.27 | 3.74 |
| F9 | 90.06 ± 2.81 | 21.67 ± 1.14 | 3.61 |
| F10 | 88.34 ± 2.47 | 22.56 ± 1.26 | 3.72 |
| F11 | 79.94 ± 2.64 | 18.23 ± 1.18 | 4.28 |
| F12 | 87.42 ± 3.18 | 15.97 ± 1.25 | 3.57 |
Table 4. Experimental responses of Plackett–Burman design matrix.
Note: *n=3, average of three determinations ± SD
The results of the batches prepared by applying PB design is given in Table no 4.
The range of the percentage yield, particle size (µ), and degree of swelling for the PB batches were found to be (76.25% to 90.06%), (15.37 to 24.14), and (3.58 to 4.29) respectively (Figure 8).
Such variation in the responses of the batches directed that the selected independent variables are significant (Table 4).
Yield (%)=+83.39+1.73 × A+4.22 × B+0.68 × C
Final equation in terms of actual factors:
percentage yield=+68.43500+0.34500 × Gum Arabic concentration+4.21500 × Glutaraldehyde volume+0.67667 × Span 80 concentration. The main factor affecting the yeild is glutaraldehyde.
Effect of the variables on the particle size of Acacia senegal gum microspheres:
Particle size=+19.30+0.65 × A-0.64 × B-2.58 × D
The R2 value was found to be equal to 0.8823, which means an agreement between the dependent and independent variables. The "Pred R-Squared" of 0.7352 is in reasonable agreement with the "Adj R-Squared" of 0.8382. And also the F-value of 19.99 proved the significance of the model (Figure 9). Final equation in terms of actual factors: particle size= +26.36750+0.13083 × Acacia senegal gum concentration-0.64250 × Span 80 concentration-2.58250 × stirring speed
Effect of the variables on the degree of swelling
Degree of swelling=+3.87+0.11 × A-0.22 × B +0.067 × D
The R2 value equal to 0.9446, which means an agreement between the dependent and independent variables. The "Pred R-Squared" of 0.8753 is in reasonable agreement with the "Adj R-Squared" of 0.9238. And, the F-value of 45.43 proved the significance of the model (Figure 10).
Final equation in terms of actual factors:
Degree of swelling = +3.78500+0.021333 × Asgum concentration-0.22000 × Glutaraldehyde volume+0.66667 × stirring speed
The main factor affecting degree of swelling is volume of added cross-linking agent (glutaraldehyde), as glutaraldehyde volume increase the degree of swelling decrease.
Micromeritic properties of Acacia senegal gum microspheres
Bulk density was found to be in the range of (0.548 to 0.579), the tapped density (0.643 to 0.703), Hausner ratio (1.1866 to 1.2258), angle of repose (28.1 to 33.8), and Carr’s index (15.73 to 18.42).
The micrometric property for the resultant microspheres shows that the powdered microspheres have good flow properties and good compressibility which allow them to be a good candidate for tablet formation or capsule filling.
Acacia senegal gum microspheres were successfully prepared, by the emulsification cross-linking method.Acacia senegal gum microspheres cross-linking was detected, by infrared spectroscopy and differential scanning calorimetry. Cross-linking conditions were studied, by Design-Expert® software (version 8, Stat-Ease). The flow properties of the microspheres were evaluated in terms of angle of repose, bulk and tapped densities Carr’s indices, and Hausner ratio. Cross-linked Acacia senegal gum microspheres with an average diameter range 15 µ to 25 µ were successfully prepared. Evaluation of the FTIR spectra and DSC curves Acacia senegal gum microspheres compared to, Acacia senegalgum as a reference, assure the occurrence of the cross-linking reaction. Light microscopy revealed that; Acacia Senegal gum microspheres were discrete. Results of micromeritic properties evaluation revealed that; the resultant microspheres powder has good flow properties.