ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Bamphitlhi Tiroesele1, Batsile R. Seletlo2, John C. Moreki3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The study determined the proximate composition of Hawk moth larvae (Agrius convolvuli L.) harvested in Mogonono village in Kweneng District of Botswana. Dry salted Hawk moth larvae (monakamongwe in Setswana) were obtained in Mogonono, about 15 km north-east of Molepolole. The standard procedures of AOAC (1995) were followed to analyze the proximate composition and mineral analysis. The Iron (Fe), zinc (Zn), calcium (Ca), phosphorus (P), copper (Cu) manganese (Mn) and sodium (Na) were determined. Proximate analysis showed that the Hawk moth larvae contained 17% ash, 58.5% crude protein, 25.1% crude fat, 9.0% crude fibre and 5.6% moisture. The mineral analysis showed that Hawk moth larvae contained 185.6 mg/kg ± 7.09 Fe, 67.4 mg/kg ± 8.87 Zn, 12.6 mg/kg ± 0.71 Cu, 18.4 mg/kg ± 0.96 Mn, 0.14% ± 0.01 Ca, 1.4% ± 0.09 Na and 1.20% ± 0.07 P. The current results showed that hawk moth larvae have nutritional benefits making it an alternative protein source in livestock and human diets.
Keywords |
| Hawk moth, mineral analysis, Mogonono, nutritional value, proximate analysis |
INTRODUCTION |
| The world population is increasing at a higher rate, implying that the food production and supply should grow at a similar rate if not faster. Therefore, it is important that cheaper sources of protein and other nutrients be established. This could be obtained from the plant materials in abundance [1] or from animals. Insects in nature constitute a significant biomass. They are mostly primary consumers and due to their high rate of reproduction, they tend to dominate all the sources of energy because of competitive exclusion [2]. The many benefits that insects offer us are often overlooked and underestimated such as their use in human and animal nutrition, in medicine, as well as in recycling of organic matter [3]. The chemical composition and nutritional value of some insects have been extensively investigated in various parts of the world [4], [5], [6]. Reference [7] reported that due to insects’ high nutritional value and ubiquitous presence, they are a potential sustainable food resource in animal nutrition. Insects have played an important role in nutrition, especially in areas where human and domestic animal populations were subjected to chronic protein deficiency [6],[8], [9]. Several research works have been done and these established a foundation of using insects as a source of protein in animal nutrition. In Botswana, some insects such as the termites, ants, hawk moth larvae and others, are often picked by chickens. The Hawk moth, Agrius convolvuli L. larva (Lepidoptera, Sphingidae) is commonly known as monakamongwe in Botswana [7]. The name monakamongwe is derived from the short upright horn [7] at the end of the abdomen. The authors stated that A. convolvuli larva which is available during the rainy season (i.e., October to January) derives its nutrition from leaves of creeping plants. The larva is also eaten by humans as a snack or side dish in Botswana. |
| There is limited information on the nutritional value of insects in Botswana. There is a need to explore more available insects for their use in human and animal nutrition. The objective of this study is to investigate the proximate and minerals composition of the hawk moth (Agrius convolvuli L.) larvae. |
MATERIALS AND METHODS |
| A. Experiment Location |
| The laboratory analysis was carried out at the Botswana College of Agriculture which is located 10 km away from the capital city, Gaborone. Samples of hawk moth larvae were collected from Mogonono for its proximate composition and mineral analysis to evaluate its nutritive value. |
| B. Experimental Procedure |
| The samples of degutted and sun-dried hawk moth larvae were collected from a local farmer in Mogonono. Degutting and sun-drying is a process of preservation of these insects. The samples upon arrival at the laboratory were kept at room temperature until the commencement of the experiment. The samples were again dried in an oven at 60- 70 °C for two days to determine the moisture content before further analysis. After drying, mortar and pestle were used for hand grinding 500 g of the hawk moth larvae samples to a powder form. All the larvae were ground together into one sample which was then divided into three portions to represent three replications for analyses. After grinding the samples, they were prepared for proximate and mineral analysis. Proximate analysis was performed to determine moisture, protein, fat, ash, and fibre according to methods of AOAC [10]. Mineral contents of the hawk moth larvae were determined by Inductively Coupled Plasma-Optical Emission Spectroscopy according to the methods of AOAC [10]. |
| C. Proximate and Mineral Analysis |
| i. Determination of moisture content |
| The ground samples were weighed and placed in an oven and heated at a constant temperature of 65 0C for 2 days. This removes the water, so loss in weight represents the water and the remaining portion is the dry matter. Drying in an oven at 60-70 0C is quite satisfactory for materials that are characteristically fibrous and starchy in nature. After drying was completed, the samples were removed from the oven and placed into a desiccator to cool to room temperature and weighed afterwards. Moisture free samples were then used for further analysis. Dry matter content was calculated using the formula below: |
 |
| ii. Crude protein analysis |
| Crude protein was analyzed using the kjeldahl method. Half a gram of dried ground sample was weighed on an analytical balance into a kjeldahl digestion flask. One gram of a catalyst mixture (Na2SO4 mixed with anhydrous CuSO4 in a ratio of 10:1) was added. Five milliliters of concentrated H2SO4was also added. The digestion flasks were then placed in the digester and the temperature was set at 3500C for 2 hours. Digestion converts any organic nitrogen compounds in the sample into ammonia and other organic matter to CO2 and H2O. After 2 hours of digestion, the flasks were then removed and allowed to cool. When cooling was complete the content in the flask was diluted by distilled water and a concentrated NaOH (40%) added to neutralize the acid and to make the solution slightly alkaline. The amino was then distillated into receiving flasks that consisted of a standardized strong acid (0.1N H2SO4) for reaction with ammonia. The excess acid was then back titrated with standard NaOH. Nitrogen % was then calculated as follows: |
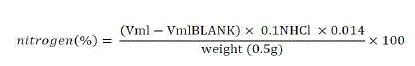 |
| The % of nitrogen was then converted to % of protein by using the conversion factor 6.25. %Protein = %Nitrogen × 6.25 |
| iii. Ash analysis |
| After the determination of the moisture content the same sample was place into porcelain dishes and then taken into the furnace at temperature of 550 0C for 4hours. The water, protein, fat and carbohydrates are completely removed by this process. When four hours elapsed the dishes were removed and cooled in a desiccator. The ash was then calculated as: |
 |
| iv. Determination of Crude fibre |
| The sample was digested with boiled H2SO4 (1.25%), then vacuum filtered and washed. The ceramic fibre was used as the filtration aid and fritted glass crucible for drying and ignition purposes. Subsequently the same sample was digested again by boiling with dilute alkali (1.25% NaOH) vacuum filtered, washed and dried. The dried residue was ignited and crude fibre was estimated as the loss in mass on ignition of the dried residue. |
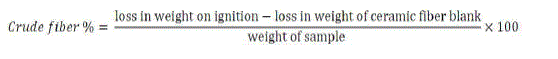 |
| v. Fat analysis |
| The lipid in fraction of a food is insoluble in water but soluble in organic solvents such as diethyl ether, petroleum spirit or a mixture of chloroform and methanol. In this analysis the total lipid content of our sample was determined gravimetrically by extraction in diethyl ether for 40 minutes at a temperature of 90 0C. When the extraction process was complete the samples were then put in an oven at a temperature of 70 0C for 30 minutes. After that the samples were then cooled at room temperature in a desiccator and weighed. The % crude fat was then calculated as below: |
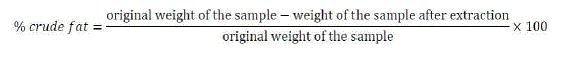 |
| vi. Mineral analysis |
| Half a gram of dried sample and 5 ml of concentrated nitric acid was added to a 50 ml folin digestion tube to prepare the samples for digestion. The samples mixtures were then digested by heating at 350 0C for 8 hours and then treated with hydrogen peroxide for 2 hours. This was then left to cool for 1 hour. After cooling, the samples were then prepared for analysis by putting them into 50 ml volumetric flasks and then diluted to the 50 ml mark. concentrations of Ca, P, Na, K, Mg and Fe were determined at specific wavelengths for each element by an Inductively Coupled Plasma- Optical Emission Spectroscopy (ICP-OES) using a Thermo Jarrell Ash IRIS instrument (Thermo Jarrell Ash Corporation, Franklin, MA). The instrument was calibrated against standards (Junsei Chemical Co., Ltd., Tokyo, Japan) of known concentration. |
| vii. Phosphorus analysis |
| The determination of phosphorus depends on the formation of phosphomolybdenum blue complex therefore phosphorus was determined by using the molybdenum blue method of Dickman and Bray. Phosphorus was complexed with acidified ammonium molybdate to form phosphomolybdate. The phosphomolybdate was then reduced to molybdenum blue by the addition of stannous chloride reducing reagent. The solutions were then read by a spectrometer and phosphorus was calculated as follows: |
RESULTS AND DISCUSSION |
| A. Proximate analysis |
| Table 1 shows the proximate analysis of hawk moth larvae.The results have revealed that the larva contains 5.6% moisture, 17% ash, 58.5% crude protein, 25.1% crude fat and 9.0% crude fiber. |
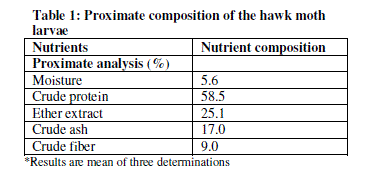 |
| The findings from this work have revealed the presence of different nutrients in the hawk moth larvae. It has been shown that the larvae have significant amounts of protein. Proteins have many fold functionalities in human and animal diet such as body building, source of energy, structural components and for other non-nutritional functionalities (gelling agents, emulsifiers, foaming agents, thickeners, stabilizers and visco-elastifiers). The relatively high protein content (58.5% on a dry basis) of the larvae is an indication that this insect can be of value in human and animal diets, particularly in developing countries where the cost and scarcity of conventional protein sources are major factors affecting production. The protein content of the hawk moth larvae compares very well with those of conventional protein feed supplements such as soybean meal (46.8%), fishmeal (60.2%) [11] and maggot-meal (63.99%) [12]. The crude protein of the hawk moth larvae also compares very well with other insect which are commonly used in animal feeds such as Phane, Imbresia belina (Westwood), at 55% [13]; field crickets, Gryllus testaceus Walker, at 58.3% [11]; grasshoppers at 60% [3] and Mormon cricket, Anabrus simplex Haldeman, at 58% [14]. The larva was higher that percentages of proteins reported for Oryctes monocerus at 36.45% [15]. Furthermore, the protein content in the larvae also proves that it can sufficiently contribute to the daily protein requirements of humans, which is about 23-56 g [16]. Generally, insect proteins are known to be of good digestibility containing some essential amino acids in appreciable amounts [15]. |
| This study has also shown that the hawk moth larvae contained high levels of fat at 25.1%. The fat content of the larvae is higher than what has been reported for the field cricket (10.3%) [11]. Reference [15] reported that insects vary widely in fat content and that Isoptera (termites) and Lepidoptera (larvae) rank among the highest in fat. The high fat content in this study, which is an indicator of high energy content potential [11] shows that the larvae of this insect has high energy than other protein supplements such as maggot meal which has fat content of 24.31% and gross energy of 5.524 Kcal/kg. The moisture content of 5.6% compares very well with maggot meal at 5.28% [12]. The ash content of hawk moth larvae of 17.0% is higher than that of fish meal (12.51%), soybean meal (6.13%), field crickets (2.96%) and Oryctes monocerus (4%) [11], [15]. Ash represents the inorganic residues in feeds thus showing the level of mineral residues such as Ca, Mg, K, Na, Fe, Zn, P and Cl. The crude fibre content in the current study was found to be 9%. This compares well with other insects such as O. monocerus at 10.5% [15] acridids (O. fuscovittata at 7.51%, A. exaltata at 7.73%, H. banian at 7.16%) [3]. It has been demonstrated, based on research conducted in recent years, that the inclusion of moderate amounts of different fibre sources in the diet improves digestive organ development [17], [18] and increases HCl, bile acids, and enzyme secretion [12], [20]. However, high crude fibre reduces food digestibility and this can consequently lead to depressed appetite [17]. |
| B. Mineral analysis |
| The mineral contents of the hawk moth larvae are presented in Table 2. It has been revealed that the hawk moth larvae contain Fe (185.6 mg/kg ± 7.09), Zn (67.4 mg/kg ± 8.87), Cu (12.6 mg/kg ± 0.71), Ca (0.14% ± 0.01), Mn (18.4 mg/kg ± 0.96), Na (1.40 % ± 0.09) and P (1.2% ± 0.07). |
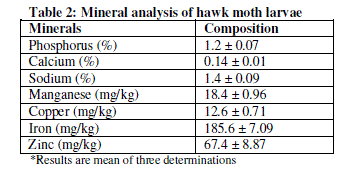 |
| The hawk moth larvae contained appreciable amounts of Ca, Fe, Na, Cu, Mn, P and Zn (Table 2) which are of immense importance to both humans and animals.Calcium is essential for bone and egg shell formation as well as muscle contraction. The present study showed that the hawk moth larvae have lower amounts of Ca (0.14%) compared to maggots with 2.01% [12], OxyafuscovittataMarschallwith 8.4%, Acridaexaltata Walker with 7%, and Hieroglyphusbanian Fabricius with 6% [3]. Lack of Ca and P in animal diets can cause broken bones and bloody meat during processing of the carcass [21]. Phosphorus plays an important role in transfer and utilization of energy. The P level of 1.2% in the present study, it compares very well with maggot meal with P of 1.32% [12]. Phosphorus is present in every living cell in the nucleic acid fraction. Phosphorus deficiency causes lower body weight, reduced feed efficiency, skeletal problems and reduced egg shell quality [21]. Zinc has been shown to be available at high amounts (67.4 mg/kg) in this study. Zinc is a trace element that is necessary for normal growth and maintenance and includes among other functions bone development, feathering, enzyme structure and function, and appetite regulation for all avian species [22]. Symptoms of Zn deficiency in poultry include suppressed immune system, poor feathering and dermatitis, infertility and poor shell quality. The hawk moth larvae had 185.6 mg/kg Iron content. Fe help red blood cells transport oxygen to all parts of the body. Reference [23] stated that Fe also contributes significantly in specific processes within the cell that produce the energy for an animal body. It is for this reason that one of the first symptoms of Fe deficiency is tiredness and fatigue [23]. Copper in this study was at 12.6mg/kg. This level is high compared with Oryctes monocerus larvae (10 mg/kg) in the study by [15]. Copper is an essential mineral required for proper bone growth and development, as well as, enzyme function [24]. In addition, Cu is often added to poultry diets at prophylactic concentrations for its growth promoting effects [25]. It is closely associated with iron metabolism as it is a part of ceruloplasmin which is an enzyme that plays an important role in the oxidation of ferrous to ferric iron, controlling the movement of iron from the reticuloendothelium to liver and then plasma, affecting red blood cell formation [24]. Deficiency in Cu can cause microcytic hypochromic anaemia and bone abnormalities due to abnormal collagen synthesis [23]. Manganese content at 18.4 mg/kg also is higher than what was shown by [15] in Oryctes monocerus larvae (12.1 mg/kg). This mineral plays a significant role in the animal’s body, particularly in the formation of chondroitin sulfate. This mucopolysaccharide is an important component of bone cartilage. Deficiencies of Mn in poultry will result in perosis, bone shortening and bowing and in poor eggshell quality in laying hens. Sodium (1.4%) on the other hand is generally the salt component in animal diets and an essential nutrient known to influence several aspects of normal animal growth. Sodium deficiency leads to reduced growth and feed consumption and impairs feed conversion. Sodium also affects water intake [26], acid-base balance [27], and basal metabolism. |
CONCLUSION |
| The present results showed that the hawk moth larva is a good source of protein and other nutrients, thus making it a potential alternative protein source in human and livestock (including poultry) diets. This study used hawk moth larvae samples from one location, therefore, further studies should be carried out from other locations. The high protein value of the hawk moth larvae suggest that this larva can be used as an alternative protein source in livestock diets. |
ACKNOWLEDGMENT |
| The authors are thankful to Botswana College of Agriculture for the laboratory assistance received from Mr C. Mpofu and Dr S. Bagwasi from the Department of Crop Science and Production, Mr F.T. Thema from the Department of Animal Science and Production and also MrGwambafrom the Department of Food Science and Technology. Our sincere thanks are extended to the Department of Agricultural Research for the laboratory assistance. |
References |
|