e-ISSN: 2319-9849
e-ISSN: 2319-9849
Jaikishor Mavai*
1Department of Chemistry, University of Delhi, New Delhi, India
Received: 18-Aug-2022, Manuscript No. JCHEM-22-72194; Editor assigned: 20-Aug-2022, PreQC No. JCHEM-22-72194 (PQ); Reviewed: 03-Sept-2022, QC No. JCHEM-22-72194; Revised: 04-Jan-2023, Manuscript No. JCHEM-22-72194 (R); Published: 12-Jan-2023, DOI: 10.4172/2319-9849.12.1.001
Visit for more related articles at Research & Reviews: Journal of Chemistry
Nitrogen (N2), Carbon Monoxide (CO), Hydrogen (H2), and carbon dioxide are the four gases that are most commonly produced during the gasification process of carbonaceous materials derived from biomass or fossil fuels. To accomplish this, the feedstock material is heated to high temperatures (typically >700°C) and subjected to a reaction in which the presence of oxygen and/or steam is controlled. Because the H2 and CO, which make up a large portion of the mixture, are flammable, the resulting gas mixture, also known as syngas (from synthesis gas) or producer gas, is also a fuel. If the gasified compounds were produced using biomass as the feedstock, then the subsequent combustion of the resulting gas is thought to be a renewable energy source. An advantage although most gasification systems produce syngas that needs to be further processed and reformed to remove impurities and other gases like CO and CO2, high temperature solid oxide fuel cells are able to accept mixtures of steam and methane directly. Syngas can also be used as the hydrogen source in fuel cells. Most frequently, syngas is used directly in internal combustion engines, utilized to create methanol and hydrogen, or transformed into synthetic fuel via the Fischer-Tropsch process. Gasification can reduce emissions of air pollutants like methane and particulates by replacing landfilling and incineration for specific materials. Some gasification methods are designed to remove corrosive ash components like chloride and potassium, enabling the creation of clean gas from otherwise trouble some feedstock materials. Electricity is presently produced on industrial sizes by the gasification of fossil fuels. Pollutants like SOX and NOX can be produced in lower quantities by gasification than by burning.
Gasification; Biomass; Technology; Carbonaceous materials; Pollutants; Combustion engines
The usage of sustainable energy has drawn more attention in recent decades, which has raised interest in the gasification process. An organic material is gasified, or thermo chemically transformed, producing syngas, a valuable gaseous product, and char, a solid result [1]. The creation of power, heat, hydrogen, and second generation biofuels may all be done efficiently through the gasification process. Since the first half of the 19th century, gasification has been used to produce energy on an industrial scale. The first public street lighting was installed in Pall Mall, London, on January 28, 1807, and it quickly spread to supply commercial gas lighting to most industrialized cities until the end of the 19th century, when it was replaced with electrical lighting. Initially, coal and peat were gasified to produce town gas for lighting and cooking. Blast furnaces and, more significantly, the production of synthetic chemicals, where it has been used since the 1920’s, continued to use gasification and syngas (Figure 1).
Numerous sites left harmful debris behind. While some locations have undergone remediation, others remain contaminated. Due to the lack of petroleum throughout both world wars, particularly World War II, the demand for gasification-produced fuel increased [2]. In Europe, gasogene, or wood gas generators, were used to power automobiles. Gasification was used to power trucks, buses, and agricultural equipment by 1945. Around the world, there were reportedly close to 9,000,000 automobiles using producer gas (Figure 2).
Access to clean and green energy has become crucial for the sustainable growth of society globally due to the rapid pace of climate change and the predicted damage caused by global warming. One of the most significant renewable energy sources to meet daily energy needs is energy, which has existed since the dawn of civilization. One of the few crucial bioenergy processes for generating heat, power, and biofuels for practical uses is gasification [3]. Despite the abundance of literature, technology, and materials, the widespread adoption of gasification technology could not get past the major obstacles preventing it from replacing conventional energy sources (Figure 3).
CO is a very valuable syngas component that can be used to create a variety of chemicals and fuels. Traditionally, coal and natural gas with fossil fuel origins which are non-renewable have been used to make syngas. CO2 gasification offers a win-win solution to the issue by simultaneously solving the problems of waste disposal and reducing carbon emissions by converting CO2 and wastes to CO. It is shown and critically reviewed how various wastes can be gasified by CO2, with a special emphasis on creating syngas that is rich in carbon. This covers the impact of advanced CO2 gasification techniques as well as operating parameters (temperature, pressure, and physicochemical characteristics of feedstocks).
Current work
Recent developments in gasification technology were the main focus of this review. Syngas production from the gasification of Petroleum Coke (PC) and the reduction of carbon emissions has garnered a lot of interest. The current paper sought to concentrate on gasification reactivity, syngas generation, and mineral transformation as they relate to demand for the gasification technique using carbon based feedstocks [4-7]. The most important variables such as feedstock kinds, temperature, gasification environment, etc. were thoroughly examined. In order to increase energy conversion efficiency and create gasification simulation models, gasification technology is used in conjunction with energy conversion performances throughout the process. To increase the microwave biomass gasification system's efficiency in using energy (Tables 1 to 4 and Figure 4).
| Reaction name | Reaction formula | ΔH298K.1atm (kJ/mol) |
|---|---|---|
| Heterogeneous reactions | ||
| Water gas primary | C(s)+H2O⇌CO+H2 | 131.3 |
| Water gas primary | C(s)+2H2O⇌CO2+2H2 | 90.2 |
| Boudouard | C(s)+CO⇌2CO | 172.4 |
| Oxidation | C(s)+O2→CO2 | -392.5 |
| Partial oxidation | C(s)+1/2 O2→CO | -110.5 |
| Methanation | C(s)+2H2→CH4 | -74.6 |
| Homogeneous reactions | ||
| Water-gas shift | CO+H2O⇌CO2+H2 | -41 |
| H2 (/Steam) reforming | CO+3H2⇌CH4+H2O | - (/+) 205.9 |
| Oxidation reactions | CO+1/2O2→CO2 | -283 |
| H2+1/202→H2O | -242 | |
| Steam reforming | CH4+2H2O⇌CO2+4H4 | 164.7 |
| C6H6O+5H2O→6CO+8H2 | 642 | |
| CO2 reforming | CH4+CO2⇌2CO+2H2 | 247 |
Table 1. Reaction of gasification process.
| Reaction process | Chemical formula | Change in enthalpy |
|---|---|---|
| Gasification with Oxygen | C+1/2O2→CO | -3,922 Btu/Ib C |
| Combustion with Oxygen | C+O2→CO | -14,111 Btu/Ib C |
| Gasification with Carbon Dioxide | C+CO2→2CO | 6,267 Btu/Ib C |
| Gasification with steam | C+H2O→CO+H2 | 4,750 Btu/Ib C |
| Gasification with Hydrogen | C+2H2→CH4 | -2,672 Btu/Ib C |
| Water gas shift | CO+H2O→CO2+H2 | -650 Btu/Ib CO |
| Methanation | CO+3H2→CH4+H2O | -3,181 Btu/Ib CO |
Table 2.Reaction process.
| Parameters | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Equipment | Temp (°C) | Pressure (barg) | Flow rate (m3/hr) | Capacity (m3) | Equipment | Temp (°C) | Pressure | Flow rate (m3/hr) | Capacity (m3) |
| Cooling water pump | 25 | I | 0.600 | - | Gas polisher reactor | 700 | 6 | 0.044 | |
| Boiler water feed tank | - | - | 0.1 | RO feed pump | - | 6.5 | 2 | - | |
| Pump | - | 8 | 0.015 | - | Quenched RO system | - | 7 | 0.5 | - |
| Boiler | 150 | 6 | 0.0082 | Water tank | - | - | 0.5 | ||
| Feeding hopper | 50- 60 | - | 0.03 | Quenched water pump | - | 6.5 | 2 | - | |
| Super heater | 350- 400 | 6.9 | 0.00196 | Steam de- super heater | 300 | 6 | - | - | |
| Fluidized bed gasifier | 700 | 6 | - | 0.044 | Water separator | 40 | 6 | - | |
| Micro filter | 700 | 6 | - | - | Air booster pump | 40 | 5.5 | 8.9 | - |
| Guard bed gasifier | 700 | 6 | - | 0.044 | Adsorption column | 40 | 12 | 5 | 0.008 |
Table 3. Gasification process.
| Gasifier type | Scale | Typical temperatures | Fuel requirements | Efficiency | Gas characteristics | Other notes | ||
|---|---|---|---|---|---|---|---|---|
| Reaction | Operating | Moisture content (%) | Flexibility | |||||
| Downdraft fixed bed | 5 kWth to 2 MWth | 1000°C (1800° F) | 800°C (1450 °F) | <20% | Less tolerant of fuel switching Requires uniform particle size Large particles | Very good | Very low tar Moderate particulates | Small scale Easy to control Produces biochar at low temperatures Low throughput Higher maintenance costs |
| Updraft fixed bed | <10 MWth | 1000°C (1800° F) | 250°C (480°F) | up to 50%-55% | More tolerant of fuel switching than downdraft | Excellent | Very high tar (10% to 20%) Low particulates High methane | Small and medium scale Easy to control Can handle high moisture content Low throughput |
| Bubbling fluidized bed | <25 MWth | 850°C (1550°F) | 800°C (1450 F) | <5 to 10% | Very fuel flexible Can tolerate high ash feedstocks Requires small particle size | Good | Moderate tar Very high in particulates | Medium scale Higher throughput Reduced char Ash does not melt Simpler than circulating bed |
| Circulating fluidized bed | A few MWth up to 100 MWth | 850°C (1550 °F) | 850°C (1550 °F) | <5 to 10% | Very fuel flexible Can tolerates high ash feedstocks Requires small particle size | Very Good | Low tar Very high in particulates | Medium to large scale Higher throughput Reduced char Ash does not melt Excellent fuel flexibility Smaller size than bubbling fluidized bed |
| Indirectly heated steam gasification | Large scale | 850°C (1550°F) | 800°C (1450° F) | Flexible | Very flexible. does not require sizing, pelletizing or drying | Excellent | High methane yield | Very high throughput Low emissions, even with high chlorine feedstocks such as MSW High capital cost |
Table 4. Gasification process condition.
Chemical reactions
At about 100°C, the dehydration or drying process starts. If the temperature is high enough, the resulting steam, which is typically mixed with the gas flow, may participate in subsequent chemical processes, most notably the water gas reaction. At temperatures between 200 and 300°C, pyrolysis (or devolatilization) takes place. Up to 70% of the weight of coal is lost due to the release of volatiles and the production of char. The procedure determines the structure and composition of the char, which will then go through gasification reactions, and is reliant on the characteristics of the carbonaceous material. A promising technology for creating sustainable syngas for use in chemical and energy applications is gasification. The sintering of biomass, low syngas output with low H2/CO ratio, and low process energy efficiency are obstacles to biomass gasification. Using a fixed bed reactor, Thermos Gravimetric Analysis-Fourier Transform Infrared Spectroscopy (TGA-FTIR), and differential scanning calorimetry, in-situ generated heat from Cato-CO2 on cellulose CO2 gasification was studied (DSC). According to experimental findings, after the power to the external furnaces was shut off, the temperature difference between the traditional CO2 gasification of cellulose and the auto-thermal biomass gasification of cellulose in the fix-bed reactor was about 20°C. In comparison to traditional gasification, the auto-thermal biomass gasification produces an H2/CO molar ratio that is approximately 5 times higher. When the CaO/cellulose mass ratio rises from 0 to 5, the gas output considerably increases from 0.29 g g1 cellulose to 0.56 g g1 cellulose [8]. The TGA-FTIR results further show that applying energy compensation of CaO carbonation to lower the gasification temperature is feasible. Additionally, DSC research demonstrates that less energy is needed to degrade cellulose due to the heat that is generated during the CaO-CO2 process (Figure 5).
The combustion process happens when the char and some volatile products combine with oxygen to primarily form carbon dioxide and trace amounts of carbon monoxide, which serves as heat for the subsequent gasification reactions. The fundamental reaction in this case is, where C is an organic molecule containing carbon. The char undergoes the gasification process when it reacts with steam and carbon dioxide to produce carbon monoxide and hydrogen [9].
The most recent global pandemic, Covid-19, is the third in the previous 20 years. Due to the use of unmanageable safety equipment and personal protective equipment kits, safety precautions have resulted in the creation of a massive amount of biological waste, including plastic garbage. Biomedical waste must be treated with an exceptional treatment process that can assist humanity in managing it by adhering to the strict environmental standards prescribed in order to protect the environment, human health, and safety. The most advantageous and effective method for treating biomedical waste is plasma gasification. The circular economy concept can be strengthened by using the generated byproducts as useful inputs in other sectors of the economy. In the current situation, plasma gasification for the treatment of biological waste has been examined. The technology's viability and application in treating biomedical waste have been examined in this study through a number of research articles. Additionally, additional procedures have been recommended for the Indian situation in order to eventually make this t echnology commercially feasible is a key technology in the shift to the "hydrogen economy" and is a significant process for the coal based hydrogen production system. A novel three step gasification technology that is thermally coupled with the chemical looping combustion process is proposed to lessen the exergy destruction and increase the cold gas efficiency of the coal gasification process. Additionally, a three-step gasification method is used to incorporate a hydrogen production system with CO2 recovery. According to the results, the three step coal gasification technology's cold gas efficiency is 86.9%, 10.1% higher than that of GE gasification technology. In addition, the novel system's 62.3% energy efficiency is 3.1% higher than the reference system's. According to an exergy analysis, using the three-step gasification technique helped to cut the system's exergy dest ruction by 4.2%. The contamination of producer gas with tar due to ineffective removal methods remains a major challenge in the bioenergy industry and a crucial barrier, impeding commercial applications of biomass gasification technology, according to the Energy Utilization Diagram (EUD), which suggested that matching between endothermic reactions and exothermic reactions plays an important role in the process. It takes more than one syngas treatment to completely remove tar using the primary and secondary tar removal methods. Plasma reforming and catalytic reforming are two tar removal technologies that are currently in use globally. Although there are drawbacks to both approaches, including rapid catalyst degradation brought on by coke deposition and decre ased syngas selectivity with significant amounts of undesirable liquid products from plasma reforming. Our review paper shown that hybrid plasma catalysis could be a significant improvement over current tar reforming techniques. However, there hasn't been much research on articles that combine heterogeneous catalyst and non-thermal plasma. An affordable and practical future technique for biomass gasification based tar reforming is plasma catalysis. The synergistic impact produced by the interaction of energ etic plasma species with catalyst radicals in tar reforming was thoroughly evaluated in the article. Review findings demonstrate that combining plasma with catalysts, particularly nickel, non-nickel metal catalyst, and zeolites, produced favourable results in terms of improved gas selectivity, tar conversion efficiency, and catalyst quality. Additionally, at the temperatures in a gasifier, the reversible gas phase water-gas shift reaction approaches equilibrium relatively quickly. The concentrations of carbon monoxide, steam, carbon dioxide, and hydrogen are all balanced as a result.
In essence, a small quantity of oxygen or air is injected into the reactor to allow a portion of the organic material to be "burned" to create carbon dioxide and energy, which fuels a subsequent reaction that transforms more organic material into hydrogen and more carbon dioxide [10].
When the newly synthesized carbon monoxide and the remaining water from the organic material react, methane and surplus carbon dioxide are produced. In reactors that increase the residence time of the reactive gases and organic components, as well as the heat and pressure, this thi rd reaction happens more frequently. In more complex reactors, catalysts are utilized to speed up reactions and bring the system closer to the reaction equilibrium for a set amount of time.
Rice hulls and other fine, unidentified biomass must be blown into the reactor by a fan in order to gasify them. As a result, very high gasification temperatures of up to 100 °C are produced. Most complex hydrocarbons are broken down into their simplest components of hydrogen and carbon monoxide as the gas is driven throu gh a bed of fine, hot char that forms above the gasification zone. The gasification agent gas flows in a co current configuration with the fuel, similar to the counter-current kind (downwards, hence the name "down draught gasifier"). The upper portion of t he bed needs to be heated, either by burning a modest amount of fuel or by using outside heat sources. The temperature of the generated gas as it exits the gasifier is high, and much of this heat is frequently transferred to the gasification agent placed o n top of the bed, giving rise to energy efficiency comparable to that of the counter-current type. Tar levels are significantly lower than with the counter-current type since all tars in this setup must pass over a hot bed of char (Figure 6).
A fluidized bed gasification factory is being built in Amsterdam with the goal of producing biofuels from garbage. In 2023, operations are anticipated. The fuel is fluidized in either air or steam with oxygen. The ash is extracted dry or in large, defluidizing agglomerates. Dry ash gasifiers operate at extremely low temperatures, necessitating the use of highly reactive fuel; low-grade coals are an excellent choice in this regard. The agglomerating gasifiers can handle higher rank coals due to their somewhat higher temperatures. The fixed bed gasifier's fuel throughput is higher than the entrained flow gasifier's, but not by as much. Due to the elutriation of carbonaceous material, the conversion efficiency may be rather poor. To boost conversion, solids might be recycled or burned again later. The best fuels for fluidized bed gasifiers are those that produce extremely corrosive ash that would harm the walls of slagging gasifiers. Corrosive ash is typically present in high concentrations in biomass fuels [11-14].
The heat and biomass distribution inside a gasifier are improved by fluidized bed gasifiers, which use inert bed material in a fluidized state. The surface fluid velocity at a fluidized state is higher than the minimal fluidization velocity needed to lift the bed material against the bed's weight (Figure 7). There are three types of fluidized bed gasifiers: Dual Fluidized Bed (DFB), Circulating Fluidized Bed (CFB), and Bubbling Fluidized Bed (BFB).
Due to concerns about landfilling, a conventional treatment, due to the increasing expansion in disposal capacity and pollution, gasification and combustion processing are deemed reliable and practical technologies. The energy conversion of the thermal treatment process is carried out in gasification and combustion processing, which also increases the effectiveness of energy recovery [15]. By taking into account the environmental and economic viewpoints, this study sought to suggest an efficient waste treatment strategy. This study offered observations on the technical aspects of the gasification and combustion processes, including the characteristics of the reactor, the media used in gasification or combustion, and the operating conditions used for the treatment of sewage sludge. This report also included a summary of the thermodynamic cycle's use in conjunction with various heat recovery techniques for the production of electricity. The research findings show that at a combustion temperature of 800-850°C, sewage sludge combustion efficiency might reach up to 99%. Under steam/oxygen gasification, the maximum Hydrogen gas (H2) content was measured at 40 mol%, and the low heating value of syngas for sewage sludge gasification was 6-7 MJ/Nm3. The combination of External Fired Gas Turbines (EFGT) without carbon capture and air gasification demonstrated the highest energy efficiency at 37.1%, above the 35.7% obtained from waste combustion technology.
Is a cutting edge renewable energy technology that has great promise for reducing dependency on fossil fuels, addressing environmental issues in long term planning, and accomplishing sustainable development objectives. Effective renewable energy laws, such as those pertaining to biomass and bioenergy, have been implemented in India. The technology of biomass gasification has many applications, including the production of hydrogen, second-generation biofuels, chemicals, and heat and power. The choice, use, and commercialization of gasification technology involve many factors. Biomass gasification has a number of advantages, including higher efficiency and fewer CO2 emissions, but it has a slow commercialization rate because of unique problems with technology, execution, and regulation. The study provides an overview of gasification technologies, including their many application pathways, techno economic viability, role in climate mitigation, and legislation, with a particular focus on biomass gasification in the Indian context.
Among the sustainable bioenergy byproducts from gasifying biomass resources are bio hydrogen and bio syngas. Even though Microwave Assisted Gasification (MAG) is still a relatively new technology, it is unquestionably a promising conversion technology for developing a sustainable bio economy. Although this technology has a great deal of potential to be fully utilized in the near future, it still requires improvements to the selectivity and efficiency of the production of syngas and bio hydrogen in order to ensure a financially and energy-efficient industrialization. This article discusses the significance of ideal operating conditions and factors in the gasification system design while providing a thorough review of the regular, microwave induced plasma, and catalytic MAG systems in relation to their production of bio hydrogen and bio syngas, carbon conversion efficiency, and tar removal. To offer factual insights for additional study and practical application in industry, a number of perspectives are also explored, including advantages, difficulties, numerical simulations, and scalable opportunities.
In a plasma gasifier, a torch is fed a high voltage current to produce a high-temperature arc. The inorganic waste is recovered as a material that resembles glass. There are numerous distinct feedstock types that can be used in a gasifier, each having unique qualities, such as homogeneity of all these attributes, size, shape, bulk density, moisture content, energy content, chemical composition, and ash fusion characteristics. The principal feedstocks for numerous big gasification plants throughout the world are coal and petroleum coke. Additionally, a variety of biomass and waste-derived feedstocks can be gasified, including switch grass, discarded seed corn, corn stover, plastics, aluminium, wood pellets and chips, waste wood, Municipal Solid Waste (MSW), Refuse Derived Fuel (RDF), agricultural and industrial wastes, sewage sludge, and other crop residues [16].
A method for the gasification of black liquor has been created by Chemrec. Compared to incineration, waste gasification provides a number of benefits: The syngas can be cleaned extensively instead of the much larger volume of flue gas produced after combustion. In comparison to the steam cycle utilized in incineration, electric power can be produced in engines and gas turbines for much less money and with greater efficiency. Even fuel cells have the potential to be used, but they have very strict requirements for the gas purity. Other synthetic fuels may be produced instead of power when the syngas is chemically processed (from gas to liquids). Heavy metal-containing ash is sometimes treated during gasification at extremely high temperatures in order to release it in a glassy and chemically stable form. A significant obtaining a suitable (positive) gross electric efficiency is a significant problem for waste gasification devices. By using a lot of pure oxygen (which is frequently used as a gasification agent) and cleaning the gas, waste preprocessing consumes a lot of power, which reduces the high efficiency of turning syngas into electric power. Getting long service intervals in the plants is a hurdle that becomes obvious when applying the processes in real life. This will prevent the need to shut down the plant for reactor cleaning every few months (Figure 8).
Environmentalists claim that gasification is nonetheless harmful to air quality and public health, calling it "incineration in disguise." According to the global alliance for incinerator alternatives, "many proposals for waste treatment facilities hoping to use...gasification technologies failed to receive final approval to operate when the claims of project proponents did not withstand public and governmental scrutiny of key claims." In Ottawa, one plant that was in operation from 2009 to 2011 experienced 29 "emissions events" and 13 "spills" during that time. Additionally, it was only functional around 25% of the time. Several waste gasification techniques have been proposed, but only a small number have been built and tested, and only a small number have been put into practice in plants that actually process garbage [17].
Since the year 2000, one factory in Chiba, Japan, employing the thermo select process, has been processing industrial waste using natural gas and pure oxygen, although the technique has not been shown to provide any net positive energy. In New Bedford, Massachusetts, Ze-gen built a waste gasification demonstration facility in 2007. The facility was created to demonstrate the use of liquid metal gasification for the gasification of particular non-MSW waste streams. This facility was built after plans for a comparable plant in Attleboro, Massachusetts, were abandoned due to strong public opposition. Ze-gen currently looks to be inactive, and the website for the business was removed in 2014 at the hurlburt field Florida special operations command air force installation, a plasma system supplied by Pyro Genesis Canada Inc. was tested in 2011 to gasify municipal solid waste, hazardous waste, and biological waste. The facility, whose construction cost $7.4 million, was shut down and sold at a government liquidation auction in May 2013. The starting offer was $25. The winning offer was locked in syngas can be utilized to generate mechanical and electrical power as well as heat. When opposed to solid fuels, producer gas offers more control over power levels, resulting in a more effective and cleans operation. Syngas can also be utilized to create liquid fuels or chemicals through additional processing. Heat gasifiers are a flexible alternative for thermal applications since they may be installed into already existing gas fueled appliances like ovens, furnaces, boilers, etc. where syngas can substitute for fossil fuels (Figure 9). The heating values of syngas typically range from 4 to 10 MJ/m3.
Currently, industrial scale gasification is primarily used to create syngas, which is then burned in gas turbines, to generate electricity from fossil fuels like coal. Utilizing Integrated Gasification Combined Cycles (IGCC), gasification is also used industrially to create electricity, ammonia, and liquid fuels (oil), with the potential to also create methane and hydrogen for fuel cells. In comparison to traditional technologies, IGCC is also a more effective way to capture CO2 . Since the early 1970’s, IGCC demonstration plants have been in operation, and some of the 1990 ’s era plants are now starting to operate commercially. New zero carbon biomass gasification plants have been insta lled in Europe that produce tar free syngas from wood and burn it in reciprocating engines connected to a generator with heat recovery. These plants are suitable for small business and building applications where the wood source is sustainable. Despite having seven distinct processes, this type of plant often referred to as a wood biomass CHP unit involves the following: The processing of biomass, the delivery of fuel, the gasification and clea ning of the gas, the disposal of waste, the generation of electricity, and the recovery of heat. Producer gas can be used to run diesel engines in a dual fuel mode. It is simple to achieve a diesel substitution of over 80% at high loads and between 70 and 80% under typical load variations. 100% gasification gas can be used to power solid oxide fuel cells and spark ignition engines. For example, driving water pumps for irrigation or coupling with an alternator to generate electricity are two uses for the mechanical energy from the engines.
Despite the fact that small scale gasifiers have been around for well over a century, it has be en difficult to find a machine that is ready to use. Small devices are frequently doing it yourself undertakings. However, a number of businesses already sell gasifiers to power small engines in the United States. Alternative fuels and energy in theory, almost any organic substance, including biomass and used plastic, can be gasified. Syngas that is produced can be burned. Alternatively, if the syngas is sufficiently clean, it could be converted effectively into Di-Methyl Ether (DME) by methanol dehydration, methane through the Sabatier reaction, or diesel like synthetic fuel through the Fischer-Tropsch process [18] . The majority of the inorganic elements, including metals and minerals, from the input material are often maintained in the ash during various gasification processes. This ash has low leaching qualities and takes on a glassy solid form in some gasification processes (slagging gasification), but expenses are hig her and net power output is low (or even negative). Regardless of the ultimate fuel type, the process of gasification does not directly emit or trap greenhouse gases like carbon dioxide. However, there is a possibility that significant amounts of electricity will be consumed during the gasification and syngas conversion processes, which c ould indirectly result in CO2 emissions; in the case of plasma and slagging gasification, this electricity consumption may even outweigh any syngas derived energy (Figure 10).
The amount of carbon dioxide released during the combustion of syngas or fuels generated from it is same to what would have been released had the fuel not been used. Conclusion gasification is a process that turns carbonaceous materials derived from biomass or fossil fuels into gases, with Nitrogen (N2), Carbon Monoxide (CO), Hydrogen (H2), and Carbon Dioxide (CO2) making up the main components (CO2). This is accomplished by regulating the amount of oxygen and/or steam present in the reaction and heating the feedstock material to high temperatures (usually >700°C) without combustion. Due to the flammability of the H2 and CO, which make up a substantial portion of the combination, the resulting gas is known as syngas (from synthesis gas) or producer gas and is itself a fuel. If the gasified chemicals were made from biomass feedstock, then the following combustion of the resulting gas is thought to be a source of renewable energy. Because syngas can be burned at greater temperatures than the original feedstock material, it has the potential to be more efficient than direct combustion. This higher temperature combustion raises the efficiency's thermodynamic upper limit, which is determined by Carnot's rule. Syngas can also be used as the hydrogen source in fuel cells, but to make it appropriate for use in low-temperature fuel cells, the syngas produced by the majority of gasification systems needs to be further processed and reformed to remove impurities and other gases like CO and CO2.
Most frequently, syngas is used directly in internal combustion engines, utilized to create methanol and hydrogen, or transformed into synthetic fuel via the Fischer-Tropsch process. Gasification can reduce emissions of air pollutants like methane and particulates by replacing landfilling and incineration for specific materials. Some gasification methods are designed to remove corrosive ash components like chloride and potassium, enabling the creation of clean gas from otherwise troublesome feedstock materials. Electricity is presently produced on industrial sizes by the gasification of fossil fuels. Pollutants like SOx and NOx can be produced in lower quantities by gasification than by burning. Since the first half of the 19th century, gasification has been used to produce energy on an industrial scale. The first public street lighting was installed in Pall Mall, London, on January 28, 1807, and it quickly spread to supply commercial gas lighting to most industrialized cities until the end of the 19th century, when it was replaced with electrical lighting. Initially, coal and peat were gasified to produce town gas for lighting and cooking. Blast furnaces and, more significantly, the production of synthetic chemicals, where it has been utilized since the 1920s, continued to use gasification and syngas. Numerous sites left harmful debris behind. While some locations have undergone remediation, others remain contaminated. Numerous sites left harmful debris behind. While some locations have undergone remediation, others remain contaminated. Due to the lack of petroleum throughout both world wars, particularly World War II, the demand for gasification produced fuel increased. In Europe, gas generators made of wood, sometimes known as gasogene or gazogène, were used to power automobiles. Gasification was used to power trucks, buses, and agricultural equipment by 1945. Around the world, it is believed that there were close to 9,000,000 automobiles using producer gas [19].
At about 100°C, the dehydration or drying process starts. If the temperature is high enough, the resulting steam, which is typically mixed with the gas flow, may participate in subsequent chemical processes, most notably the water-gas reaction. At temperatures between 200 and 300°C, pyrolysis (or devolatilization) takes place. Up to 70% of the weight of coal is lost due to the release of volatiles and the production of char. The procedure determines the structure and composition of the char, which will then go through gasification reactions, and is reliant on the characteristics of the carbonaceous material. The combustion step happens when the char and some volatile products combine with oxygen to predominantly generate carbon dioxide and trace amounts of carbon monoxide, which serves as heat for the subsequent gasification events. The basic reaction here is the gasification process, which takes place as the char combines with steam and carbon dioxide to form carbon monoxide and hydrogen. Let C represent a carbon containing organic substance. Additionally, at the temperatures in a gasifier, the reversible gas phase water-gas shift reaction approaches equilibrium relatively quickly. The concentrations of carbon monoxide, steam, carbon dioxide, and hydrogen are all balanced as a result. In essence, a small quantity of oxygen or air is injected into the reactor to allow a portion of the organic material to be "burned" to create carbon dioxide and energy, which fuels a subsequent reaction that transforms more organic material into hydrogen and more carbon dioxide. When the newly generated carbon monoxide and the organic material's remaining water mix to produce methane and more carbon dioxide, additional processes take place. Reactors with longer residence times for the reactive gases and organic materials, as well as higher temperatures and pressures, produce reactions more frequently (Figure 11). Catalysts are employed in more complex reactors to speed up reactions and bring the system closer to the reaction equilibrium for a predetermined residence period [20].
Gasification process conversion, yield, selectivity
Figure 11. Selectivity of the primary gas composition derived from the catalytic gasification of sewage sludge by the catalyst of (a) Ni 0.05 Fe 0.05 /HC; (b) Ni 0.1 /HC; (c) Ni 0.25 Fe 0.25 /HC and (d) Ni 0.5 /HC at varied gasification temperatures ranging from 500 to 900°C.
A lab fixed-bed reaction setup with an on-line quadruple mass spectrometer was used to execute potassium catalyzed steam gasification of petroleum coke for the generation of H2. In comparison to non-catalytic gasification, the gasification reactivity, selectivity, and gas release for the catalytic gasification were evaluated. The water-carbon reaction, the water-gas shift reaction, and the methane steam reforming reaction could all be effectively promoted by catalytic gasification, which could also significantly increase the gasification's selectivity toward CO2. To better understand the catalytic behaviours of catalysts, a quantitative calculation approach for the gasification selectivity towards CO and CO2 was presented. There may be a best gasification temperature (around 750°C) for producing hydrogen from the steam gasification of petroleum coke, which is catalyzed by potassium (Figure 12). In the case of catalytic gasification, the gasification temperature had opposite effects on the gasification reactivity and the gasification selectivity towards CO2. Additionally, it is feasible to use petroleum coke as the feedstock for the catalytic steam gasification to create gases with a high H2 content (55.5–60.4%) and almost negligible CH4 (below 0.1%).
One of the crucial operating factors in biomass gasification is the equivalence ratio. For biomass chemical looping gasification, the oxygen carrier's high producer gas generation over a wide range of equivalence ratios (oxygen carrier/biomass ratio) make it the ideal fuel source (CLG). This study examined, in a fixed bed reactor, the impact of various equivalency ratios on the CO selectivity of Fe2O3, Fe2O3/CaO, and CaFe2O4 oxygen carriers in biomass char CLG. As the equivalency ratio increased from 0.5 to 3.0 at 900°C and 1000°C, respectively, the results showed that the CO selectivity of CaFe2O4 had little change and declined from 70.39% to 59.79% and 73.40% to 63.98%. Contrarily, the CO selectivity of Fe2O3 changed significantly, declining from 65.86% to 31.13% (900°C) and from 69.68% to 47.27% (1000°C), respectively. Fe2O3/CaO demonstrated a tendency similar to Fe2O3 at 900°C but similar to CaFe2O4 at 1000°C. The three oxygen carriers showed high CO production at lower equivalence ratios, with CaFe2O4 showing the best CO selectivity performance over the specified equivalence ratio range. The oxygen carriers were transformed into different iron phases with various equivalent ratios, as demonstrated by X-Ray Diffraction (XRD) spectra. At the equivalence ratios of 0.5 and 1.0, the Fe (III) in the three oxygen carriers was completely converted to Fe (0), while as the equivalence ratio was raised further, FeO and Ca2Fe2O5 became the dominant species in the spent oxygen carriers (Table 5). The thermodynamic study of the trends of CO selectivity for three oxygen carriers agreed with the experimental findings.
| Model | Governing equation | Remarks |
|---|---|---|
| Volumetric reaction model | 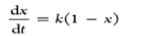 |
Reaction takes place in the entire volume |
| Shrinking core model | 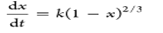 |
Reaction takes place on the surface and the particle shrinks as the reaction progresses |
| Random capillary model | 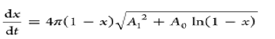 |
A1 and A2 are empirical constants |
| Random pore model | 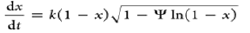 |
'Ψ' is a structural parameter |
| Discrete random pore model | 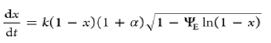 |
ΨE is the effective structural parameter and α is a discreteness parameter |
| Modified discrete random pore model | 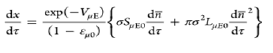 |
Takes into account different initial surface reactivity |
| Modified volumetric model | 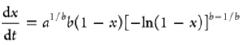 |
a and b are empirical constants |
| Dutta and Wen model | 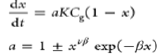 |
a is the ratio between the available surface area and the initial surface area per unit weight |
| Johnson model | 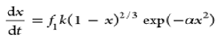 |
f1 is the relative reactive factor and αx2 is the influence of the effective surface area |
| Unification theory model | Grain model, random pore model, and any model can be applied | When conversion is plotted against dimensionless time τ = (t/t0.5), all gasification data falls on a single master curve up to conversion levels <70% |
Table 5. Gasification process.
Gasification process governing equation table
Globally, there is growing interest in using biomass to generate heat, power, liquid fuels, hydrogen, and value added chemicals while emitting less greenhouse gases. A promising approach for using biomass that has a beneficial environmental impact is gasification. This review primarily addresses recent developments in the gasification of woody biomass. The handling, pre treatment, and performance of gasification are significantly influenced by the biomass's physical characteristics, chemical composition, and structure. In order to improve the conversion and cracking of tars, primary and secondary catalysts are crucial, and lime enhanced gasification advantageously combines gasification with CO2 capture. The reaction mechanisms and biomass characterization are covered in this article. To clarify the ideas, procedures, and traits of woody biomass gasification and to pinpoint difficulties, experimental research and industry experience are examined. In addition to reviewing the historical evolution of gasification, this work also makes comparisons between gasification and combustion in a single source. It also includes a brief description of the combined cycle and integrated gasification processes. The paper's main goal is to detail the twelve main gasifiers that are currently on the market. While some of these are in various stages of development, others are already fully developed. When employing a range of fuels under varied situations, from air blown to oxygen blasted and atmospheric pressure to several atmospheres, the hydrodynamics and kinetics of each are evaluated along with the most likely gas composition from each of the technologies (Table 6).
| Reactions | Case 1 | Case 2 |
|---|---|---|
| R3 | 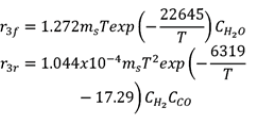 |
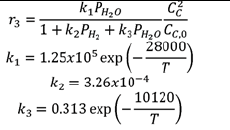 |
| R4 | 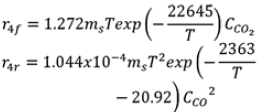 |
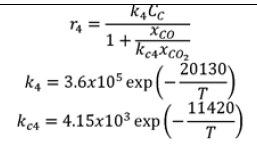 |
| R5 | 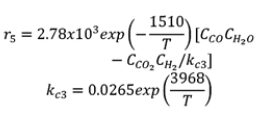 |
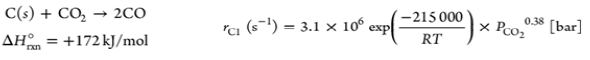 |
Table 6. Gasification process kinetic modelling.
Gasification process kinetic modelling
Based on the mechanism of surface reactions, a kinetic model for the gasification of biomass is created. By reducing the discrepancies between experimental data and theoretical conclusions for various residence durations and temperatures, the apparent rate constants are calculated. The theoretical results for various equivalency ratios are compared to experimental data to validate the kinetic model, and the simulations show good agreement with the experimental data. The results of experiments conducted by other researchers are consistent with simulations that simulate the effect of char particle size on the amount of time needed to accomplish 90% carbon conversion. The following parameters are simulated to determine how they would affect the gasification of biomass: (a) oxidant type, (b) residence duration, (c) char particle size, (d) temperature, (e) pressure, (f) equivalency ratio, and (g) moisture. The kinetics of biomass gasification in bubbling fluidized beds has been mathematically modelled. It considers two phases a bubble phase and a dense phase but is one dimensional because it can forecast temperature and concentration gradients along the reactor axis. The model also incorporates mass transfer between the two phases, a quantitative calculation of the local bubble and particle characteristics, and reaction kinetics in the dense phase. There has also been a theoretical optimization with regard to ER, pressure, bed height, and gas velocity. A comparison with experimental data from the literature was conducted at the end, and the results were mainly good, though additional validation is still needed (Table 7).
| Gasification reaction | Reaction kinetic rate expression |
|---|---|
| Boudouard | 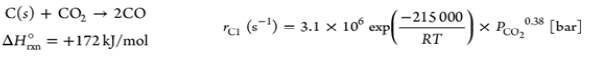 |
| Water gas | 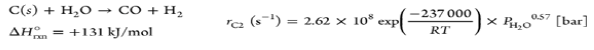 |
| Methanation | 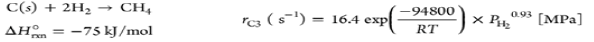 |
| Steam Methane Reforming (SMR) | 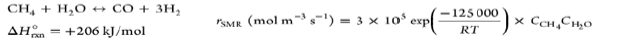 |
| Water Gas Shift (WGS) | 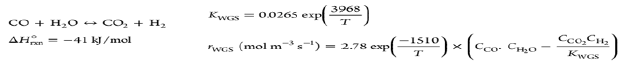 |
Table 7. Gasification process of kinetic rate expression.
A promising approach to enable resource exploitation of biomass waste is Super Critical Water Gasification (SCWG). To direct the advancement and expansion of this technology, both experimental and modelling studies are conducted. The reactor's dynamic study is a key component of these studies. However, there isn't much published material on the topic. In this study, a one dimensional dynamic model was created, and using the lumped parameter approach, a kinetic model of soybean stem was produced. This model includes a detailed reaction pathway of biomass. It was determined by fitting experimental data (obtained at three different temperatures of 600, 650, and 700°C and a pressure of about 25 MPa), after which it was transferred to the tubular reactor for additional examination. The reactor's sensitivity analysis revealed that the H2 mole fraction was most sensitive to the inlet temperature, increasing or decreasing by about 10% when the temperature fluctuated by less than 5%. According to the results of a dynamic simulation, the gasifier reacts fast to a temporary change in concentration. At 500 seconds, gasification efficiency dropped from 83.8% to 41.9%, remained unchanged for 240 seconds, then rose to 83.4% and remained steady at this level. Response time took 900 s in total. Molar fractions of CO, CO2 and CH4 all increased, correspondingly by 0.8%, 0.66% and 3.08%. H2's value dropped by 4.54% in 660 seconds. Regarding flowrate variability, gasification efficiency rose 3.4% when residence time went from 527 to 584 seconds.
The technology of biomass gasification is developing, hence additional modelling study must be done in addition to actual work. The pyrolysis process is modelled as an instantaneous process, although in the past all the attention has been focused on the combustion and reduction stages to be the governing processes. In this paper, a new improved model for the downdraft reactor's gasification process is put forth with a more accurate depiction of the pyrolysis stage as a series of temperature dependent gas releases. The proposed model, which was created using the Aspen Plus software programmer, uses kinetic control to direct the evolution of the pyrolysis gas, which is followed by the combustion and reduction reactions. A MATLAB and Aspen Plus model that is integrated carries out the simulation of the reactor temperature profile and the evolution of the pyrolysis gas. The suggested model has been tested using experimental data from the gasification of several woody biomass types while taking a variety of scale reactor and power loads into account. The results of the sensitivity analysis can be used to forecast the performance of a gasifier at various load levels corresponding to the air flow rate range of 3–10 L/s with confidence because the anticipated results are in very excellent agreement with the experimental data. Although the LHV falls as the supplied air flow rate rises, the gas yield reacts in the opposite manner, maintaining the cold gas efficiency is maintained at a good level of energy conversion at ≥ 70%. Additionally, the variability in biomass moisture content, which typically ranges from 5-25%, has a big impact on gasification efficiency. Such that high moisture content biomass significantly lowers the CO content and, as a result, the LHV of the generated gas (Figure 13). Therefore, it's crucial to keep the moisture content at the lowest possible amount.
Gasification modelling Aspen software
For the co gasification of biomass and polymers in a fluidized bed reactor employing Aspen Plus with kinetic based reactors, a thorough process model was created. To run the simulation, governing kinetic expressions from the literature were nested in the Aspen Plus programmer. In this work, sawdust is co-gasified with Poly Ethylene (PE) and Poly Propylene using steam (PP). It aims to evaluate the synergistic effects of lignocellulose biomass with PE and PP (0–30%) on the composition of syngas, hydrogen to carbon monoxide ratio (H2/CO), and Higher Heating Value (HHV). Discussed are the effects of process variables such as reactor temperature, Steam/Feedstock ratio (S/F), plastic content, and pressure. Higher hydrogen concentration in the syngas as a result of primary and secondary reforming processes is caused by an increase in the plastic component. At 30% plastic content and 750°C, the highest hydrogen concentrations of 65.32% and 63.80% were obtained for the gasification of PE biomass and PP biomass, respectively. The rise in plastic content combined with a decrease in oxygen content in the feedstock resulted in a reduction in CO, which in turn causes an increase in H2/CO. As the gasification temperature was raised, higher HHV syngas and higher hydrogen content were produced. At 800°C, the maximal hydrogen contents for the co gasification of PE and PP were 61.99% and 60.57%, respectively. The current study examines the use of a biomass gasifier to process used poultry litter pellets to produce enriched hydrogen syngas. Aspen Plus was used to model the biomass gasifier, and the model was validated using four distinct types of agricultural waste biomass in conjunction with the results of an experiment. The impact of the Equivalence Ratio (ER), gasification temperature, and moisture content on the Producer Gas (PG) composition, gas yield, H2/CO ratio, Cold Gas Efficiency (CGE), and Higher Heating Value (HHV) were examined by sensitivity analysis. RSM has also been used to optimize the variable gasification parameters across many targets. For H2, CGE, and HHV, the R2 values from the Anova are 92.26%, 93.2%, and 92.4%, respectively (Figure 14). The optimal H2, CGE, and HHV values are 0.21, 57.67%, and 5.71 MJ/Nm3 at a temperature of 830.30°C, an equivalence ratio of 0.2, and a moisture content of 16.36%, respectively. The obtained desirability value was roughly 0.87.
Recently, there has been a noticeable increase in the energy acquisition from renewable energy sources to encourage carbon neutrality. The current study aids in the creation of a trustworthy numerical model for the gasification of woody biomass into syngas using steam and CO2. To examine the effects of crucial factors including gasification temperature, reaction temperature, and gas agent composition on H2 and CO concentrations, CO and CO2 conversion, H2/CO ratio, and syngas process efficiency, Aspen Plus process simulator was used. At 900°C, the gasification system's energy efficiency was assessed. The results of the simulations demonstrated that the substitution of CO2 for H2O did not yet significantly affect the gasification efficiency, improved the energy content of the biofuel produced from the biomass, and made it easier to adjust the H2/CO ratio for subsequent synthesis. The production of valuable syngas for use in downstream synthesis applications and the reduction of greenhouse gas emissions can both be achieved by replacing H2O with CO2. Without the need for time consuming, expensive, and labor intensive experimental research, this data information can help to comprehend and optimize the overall gasification process for evaluating potential CO2 usage. Aspen Plus is used to create a numerical simulation model of the air gasification of rice husks in order to test the viability of creating hydrogen rich syngas. The results of rice husk gasification and other published investigations are used to experimentally validate the model. It was investigated how temperature and the equivalency ratio affected the composition of syngas, H2 yield, LHV, H2/CO ratio, CGE, and PCG. To find the optimal operating point for maximum H2 yield and PCG, the synchronized impacts of temperature and ER are also investigated using RSM. The RSM study results indicate that performance is best between 820 and 1090°C, with ER between 0.06-0.10. The results demonstrate that by combining simulations with sophisticated optimization approaches, the gasification system’s ideal operating conditions can be attained with greater precision (Figure 15).
To prevent upcoming global energy and environmental catastrophes, researchers are concentrating their efforts on renewable energy sources. Biomass can be processed utilizing a variety of methods and thermodynamic cycles, making it one of the renewable energy sources. For instance, biomass gasification has a number of technological and environmental advantages. On the other hand, bio char is created by applying a thermal pre treatment method to biomass, and it can be utilized to get around the limitations of biomass in gasification processes. Even though bio char increases the quantity and quality of syngas, there aren't many studies on the subject in the literature.
Although researchers have employed a variety of process simulators, the Aspen HYSYS simulator has only been utilized to build a very small number of gasification systems, and to our knowledge, no Circulating Fluidized Bed (CFB) gasification model for biochar gasification has ever been published. The non-stoichiometric equilibrium technique was used in this study to create a novel CFB gasifier model. On the syngas, the effects of various operational parameters were assessed. Additionally, in a steam and CO2 mixture, the gasification performance of 10 biochar samples with various physicochemical characteristics was contrasted with the physicochemical properties of solid fuels. As a result, during steam gasification, bio char samples with high carbon content produced an H2-rich syngas with a high calorific value. According to parametric studies, the ideal gasification temperature is around 700°C, the ideal gasification pressure is 1 bar, and the ideal steam/biochar and CO2/biochar ratios are, respectively, between 0.20 and 0.30 and 0.50 and 1.0. In this work, a fluidized bed gasifier was used to air gasify Napier grass, and a thermodynamic equilibrium model was created using Aspen Plus for parametric evaluation of syngas composition and product production. Temperature, pressure, Equivalence Ratio (ER), and moisture content were all factors that were controlled and analyzed while being further validated by experimental results. M-cresol and heptane were used as representative species in the analysis of the tar cracking process. In MATLAB, conversion of homogeneous processes was empirically correlated and then used to modify the composition of CO, H2, and CH4 for more accurate experimental data prediction, with an average mean error of 0.20 to 0.25. The greatest CH4 composition helped to reach the maximum Lower Heating Value (LHV) at 750°C. Although the ER modification had no impact on the production of bio liquids, it significantly decreased the yield of biochar (Figure 16). By encouraging the generation of CO, H2 and CH4 while reducing the yield of bio char and bio liquid, lowering moisture content greatly enhanced syngas quality. At T=750°C, ER=0.2, and moisture content of 4.5 wt%, the optimum LHV were 7.69 MJ/Nm3.
Here, an Integrated Plasma Gasification Combined Cycle (IPGCC) power plant's steady-state model development is discussed. The power generation unit, syngas conditioning units, and plasma gasifier are all included in the model. Additionally, each component model used in Aspen Plus is thoroughly documented (thermodynamic method, chemical reactions, and operative conditions). By comparing the plasma gasification results with experimental and numerical data from the literature, the proposed model was confirmed; the relative error was, respectively, 6.23% and 5.24%. Following that, a two part sensitivity analysis was conducted using the model. Municipal solid waste (MSW) simulations with moisture contents ranging from 20% to 60% were run in the first section. The increase in moisture content resulted in a 53% reduction in torch specific power usage. However, since the MSW moisture content rose from 20% to 60% as a result of the rising specific fuel consumption, the IPGCC power plant's thermal efficiency also fell by 28%. In the second section, it was found that using high plasma temperatures (5000°C) and low gasification temperatures (2000°C) allowed the IPGCC power plant to operate at its best efficiency (32.5%). The IPGGC power plant, which can produce 1000 t/day, generated 62 MW of net power under these conditions of maximum efficiency. This work proposes a trustworthy model that may analyses the sensitivity of the downdraft gasification linked to a hydrogen production unit using the Aspen Plus process simulator. The effects of important variables, such as the gasification temperature and Steam to Biomass Ratio (SBR), on the composition of syngas, its calorific value, and hydrogen production are reviewed before the ideal circumstances for the highest hydrogen production are determined. Experimental and other modelling data are used to validate the model, which is shown to be highly congruent. According to the results of the sensitivity analysis performed using simply air as the gasification agent, higher temperatures are advantageous for a product gas that has a greater hydrogen content and calorific value. Additionally, using steam as a gasifier raises the hydrogen content and heating value of the syngas compared to the use of air as gasification agent. Finally, the findings demonstrate that 80°C for the gasified temperature and 0.6 for the SBR are necessary for the sawdust downdraft gasification to produce the highest value of hydrogen.
Carbonaceous materials derived from biomass or fossil fuels can be gasified to produce a variety of gases, with Nitrogen (N2), Carbon Monoxide (CO), Hydrogen (H2), and Carbon Dioxide constituting the major portions (CO2). By regulating the amount of oxygen and/or steam present in the process, the feedstock material is heated to high temperatures (usually >700°C) and subjected to a reaction without combustion. Due to the flammability of the H2 and CO, which make up themajority of the gas, the resulting gas mixture also known as syngas (from synthesis gas) or producer gas is also a fuel inand of itself. If the gasified chemicals were made from biomass feedstock, the following combustion of the resulting gasis thought to be a renewable energy source and can be used to generate power. Because syngas can be burned at greatertemperatures than the original feedstock material, it has the potential to be more efficient than direct combustion. Thishigher temperature combustion raises the efficiency's thermodynamic upper limit, which is determined by Carnot's rule.Although most gasification systems produce syngas that needs to be further processed and reformed to removeimpurities and other gases like CO and CO2, high-temperature solid oxide fuel cells are able to accept mixtures of steamand methane directly. Syngas can also be used as the hydrogen source in fuel cells. Most frequently, syngas is useddirectly in internal combustion engines, utilized to create methanol and hydrogen, or transformed into synthetic fuel viathe Fischer-Tropsch process. Gasification can reduce emissions of air pollutants like methane and particulates byreplacing landfilling and incineration for specific materials. Some gasification methods are designed to remove corrosiveash components like chloride and potassium, enabling the creation of clean gas from otherwise troublesome feedstockmaterials. Electricity is presently produced on industrial sizes by the gasification of fossil fuels. Pollutants like SOX andNOX can be produced in lower quantities by gasification than by burning. Recent developments in gasification technologywere the main focus of this review. Syngas production from the gasification of Petroleum Coke (PC) and the reduction ofcarbon emissions has garnered a lot of interest. The current paper sought to concentrate on gasification reactivity,syngas generation, and mineral transformation as they relate to demand for the gasification technique using carbonbased feedstock’s. The most important variables such as feedstock kinds, temperature, gasification environment, etc.were thoroughly examined. In order to increase energy conversion efficiency and create gasification simulation models,gasification technology is used in conjunction with energy conversion performances throughout the process. To increasethe microwave biomass gasification system's efficiency in using energy.
[Crossref] [Googlescholar] [PubMed]