ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Mohamed Raffic.A1 Velammal Engineering College, Surapet, Chennai, India1 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
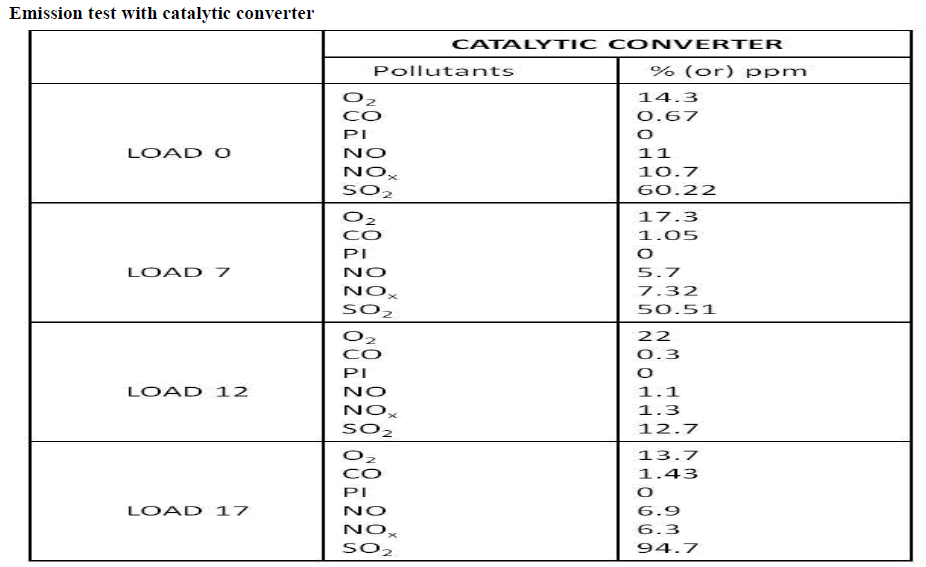
Diesel engines are widely used in many areas like automobiles, locomotive marine engines power generations etc., due to its high power output and thermal efficiency. Even though the diesel engines give more benefits, the human discomforts caused by the pollutant emission of these engines have to be considered. The major pollutant emissions of the diesel engines are particulate matters, smoke and the oxides of nitrogen (NOx). Out of these pollutant emissions, the oxides of nitrogen are considered as the most harmful pollutants to the human health. Emissions of nitrogen oxides (NOx) contribute seriously to air pollution, which is a major environmental problem. Emissions of NOx react with moisture in the air to form nitric acid, contributing to soil and water acidification in sensitive areas. There are many techniques being tried to control NOx emission from diesel engine. In this project, the emissions controlled by after treatment of exhaust gases. In the after treatment method, urea solution is dipped in steel mesh. Then the zinc oxide is coated in steel mesh. The zinc oxide acts as catalyst and converts the oxides of nitrogen (NO and NO2) into free nitrogen (N2) and water vapour (H2O).
INTRODUCTION |
| Internal combustion engines generate undesirable emissions during the combustion process. In this, both SI and CI engines are equally responsible for the same. The emissions exhausted into the surroundings pollute the atmosphere and cause serious problems. The major causes of the emissions are non-stoichiometric combustion, dissociation of nitrogen, and impurities in fuel and air. The emissions of concern are: unburnt hydrocarbons (HC), oxides of nitrogen (NOx), oxides of carbon (COx), oxides of sulphur (SOx), and solid carbon particulates. It is the dream of the engineers and scientists to develop engines and fuels such that very few quantity of harmful emissions are generated, and these could be let into surroundings without a major impact on the environment. |
CONTROL TECHNOLOGIES OF NOX |
| Many methods are available for control of NOx emissions. They are mainly classified into three categories: |
| •Control before combustion, |
| •Control during combustion process |
| •Control after combustion (post combustion control technique). |
| In case of after-treatment it consists of mainly of the use of thermal or catalytic converters and particulate traps. Diesel (compression-ignition) engines can be run at higher compression ratios, which result in higher thermal efficiencies compared to gasoline (spark-ignition) engines. As a consequence, diesel engines can reduce greenhouse gas emissions based on the same mileage driven in comparison to gasoline engines. One challenge for the diesel engine is the removal of NOx (nitrogen oxides) from the exhaust, which is a major source of acid rain and chemical smog. It can also cause respiratory problems for people. The selective catalytic reduction (SCR) or catalytic converter of NOx is a promising technology for NOx reduction. |
II. OBJECTIVE |
| The main objective is design the monolithic structure in a manner that it ensures the following, |
| •The material cost in constructing the monolithic structure should be minimized. |
| •The structure should not be bulkier, it should be in a manner that it can be easily installed in the exhaust system of an automobile. •The structure should not add much weight to the vehicle to maintain the fuel efficiency. •The monolithic structure should not create back pressure and affect the performance of the engine. The second objective is to select the catalyst that is to be coated on the monolithic structure such that it ensures the following, •The catalyst used must be cheaper than the conventional catalyst, platinum. •The catalyst should not be emptied sooner that it requires frequent re-coating. •The catalyst should not change its physical and chemical properties when subjected to high temperatures, i.e., it should withstand high temperatures. •The catalyst must not form any new pollutants during the process as by products. |
ZINC OXIDE |
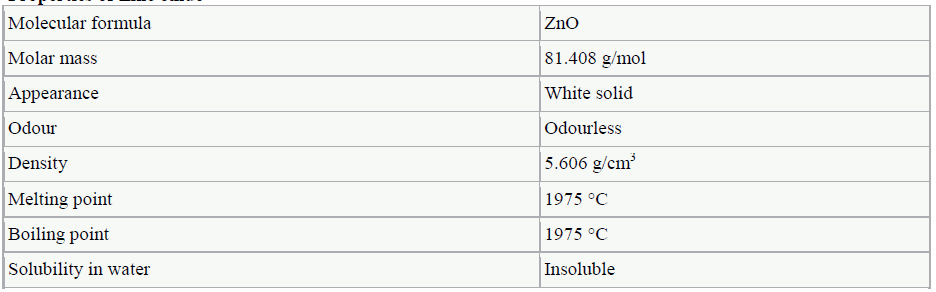 |
Production of zinc oxide |
| Indirect process In the indirect or French process, metallic zinc is melted in a graphite crucible and vaporized at temperatures above 907 °C (typically around 1000 °C). Zinc vapour reacts with the oxygen in the air to give ZnO, accompanied by a drop in its temperature and bright luminescence. Zinc oxide particles are transported into a cooling duct and collected in a bag house. This indirect method was popularized by LeClaire (France) in 1844 and therefore is commonly known as the French process. Its product normally consists of agglomerated zinc oxide particles with an average size of 0.1 to a few micrometers. By weight, most of the world's zinc oxide is manufactured via French process. |
| Direct process The direct or American process starts with diverse contaminated zinc composites, such as zinc ores or smelter byproducts. The zinc precursors are reduced (carbothermal reduction) by heating with a source of carbon such as anthracite to produce zinc vapour, which is then oxidized as in the indirect process. Because of the lower purity of the source material, the final product is also of lower quality in the direct process as compared to the indirect one |
| Wet chemical process A small amount of industrial production involves wet chemical processes, which start with aqueous solutions of purified zinc salts, from which zinc carbonate or zinc hydroxide is precipitated. The precipitate is then filtered, washed, dried and calcinated at temperatures around 800 °C. |
| Ordinary white powdered zinc oxide can be produced in the laboratory by electrolyzing a solution of sodium bicarbonate with a zinc anode. Zinc hydroxide and hydrogen gas are produced. The zinc hydroxide upon heating decomposes to zinc oxide. |
| Zn + 2 H2O → Zn(OH)2 + H2 |
| Zn(OH)2 → ZnO + H2O |
PROCESS |
| The steel wire mesh is first dipped into the hydrochloric acid solution for 1 hour to remove the unwanted materials in steel wire mesh. Then it is dipped in urea solution for 5 mins. The urea solution gives grip to steel wire mesh. Then steel wire mesh is coated by Spray Pyrolysis Chemical Coating Process |
COATING TECHNIQUES |
 |
THIN FILM |
| Thin films are thin material layers ranging from fractions of a nanometer to several micrometers in thickness |
| Based on thickness, thin films are subdivided into |
| i) Ultra-thin films - few A o to 100 Ao. |
| ii) Thin (or) very thin films - 100Ao to 1000Ao. |
| iii) Thin film - 1000Ao to 10μm and more. |
NUCLEATION |
| The growth process is consisting of a statistical process of nucleation, surface diffusion, controlled growth of the three dimensional nucleuses, formation of a network structure and its subsequent filling to give a continuous film. Depending on the thermodynamic parameters of deposition and substrate surface the initial nucleation and growth stages may be described as a island type, cluster stage, channel stage and continuous stage. |
III. GROWTH OF THIN FILM |
 |
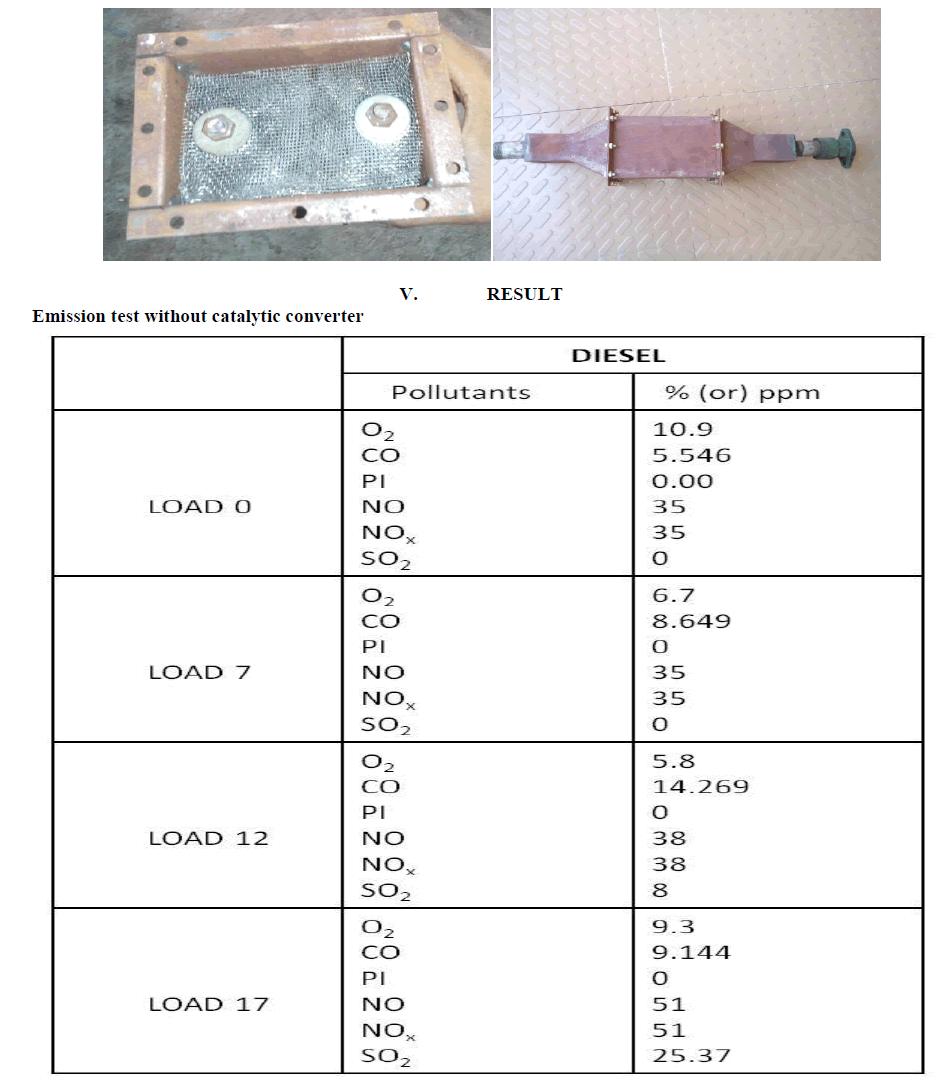
IV. EXPERIMENTAL SETUP |
| This design is made up of steel mesh method. In this substrate design it consists of 20 plates .All the steel mesh are joined by the bolt. This designed to reduce the cost of the setup and to reduce the back pressure created and to reduce NOx . Because in SCR setup so this will create a back pressure inside the reactor. And there is about 7 mm gap between the two plates |
 |
 |
VI. CONCLUSION |
| By referring through many papers and guidance, the efficiency of selective catalytic reduction can be expected to increased by combination of both zinc oxide chemical. By increasing the concentration of zinc oxide, NOx can be reduce. These can be analyse by utilizing the various concentration of Zinc oxide in selective catalytic reduction technology. zinc oxide the binder to increase the separation process |
VII. FUTURE WORK |
| The zinc oxide(ZnO) coating is removed from the wire mesh. And then the wire mesh is again coated by using barium chloride as a catalyst with the help of spray pyrolysis process. Emission test is carried out by using barium chloride as a catalyst. |
References |
|