ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Hegde Rashmi Shridhar1, Mala. J1, Manishchandra1, Bharath. S1, Jagadish H Patil2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The powdered flame of forest (Butea monosperma) seeds and its activated carbon obtained by pyrolysis was used as biosorbent for the removal of hexavalent chromium from aqueous solutions. The effect of parameters such as the initial metal ion concentration, pH, adsorbent dosage and temperature has been considered in batch tests. More than 99% removal of hexavalent chromium was achieved using crude flame of forest seeds as biosorbent and pyrolysed. The biomass flame of forest seed and carbon obtained by pyrolysing the seeds showed solid capacity of adsorbing chromium from the aqueos solution which emphasizes its importance for the chromium removal. High chromium removal is possible at low pH of about 1, high adsorbent dosage and low initial concentrations. Further, highest chromium removal was possible at moderate temperature of 400C.
Keywords |
| Hexavalent chromium, Diphenylcarbazide, Biosorption, Pyrolysis. |
INTRODUCTION |
| Growth in the industrialisation is leading to tremendous problem in the disposal of heavy metals and thus removal of these heavy metals is one of the most authoritative aims for industry. They are available in every possible area of domestic usage from structural materials to beautifying items, pharmaceutical drugs to synthetic foods; sources of fuel to agents of destruction; gadgets to personal care products. Today, the drinking water, air and soil to which many people are exposed consist of considerable amount of heavy metal. Distinctive of organic pollutants, most of which is sensitive to biological degradation, heavy metal ions do not decompose into harmless end products. Hence there is a great requirement to re-evaluate our day to day routine and the way we use our water resources. Different alternatives which can be used for handling this kind of waste water are explained in different writings, including membrane separation process, ion exchange, chemical precipitation and carbon adsorption among many other alternatives. The hunt for new engineering technique to remove harmful metals from waste-water has given a way for biosorption, which is based on trapping of metals in the biological materials. Biosorption is a rapid and a new technology to remove these heavy metals using waste biological materials such as biomass, seeds, microorganisms etc, [1]. The methods already in use for removal of heavy metals from waste water, but they are not cost effective having deficient efficiencies at low metal concentrations. Some of these methods bring forth toxic sludge. The disposal of such a toxic waste is a load on the technological and economic doability of treatment process [2]. These restrictions have caused the search for technologies which are new and a better option for metal binding to cost effective environmental satisfactory levels. The ability of biological materials to adsorb metal ions has standard substantial attention for the development of a clean, efficient and affordable engineering technique for waste water treatment at low metal concentrations [3]. |
| Biosorption is the solution for a lot of problems faced by the industries to remove hexavalent chromium. The significant advantage of biosorption over conventional treatment methods involves: high efficiency, low cost, less amount of sludge formation and the possibility of metal removal. The search for a cheaper substitute proves that utilising natural processes and materials can substantially reduce the cost of the treatment processes [4]. |
II. MATERIALS AND METHODS |
1. Preparation of Biosorbent: |
| 1.1 Collection of flame of forest seeds: Naturally available waste biosorbent flame of forest seeds collected from R.V.C.E campus, Bengaluru, Karnataka were used for batch studies for removal of chromium. Natural biosorbent along with pyrolysis was employed for comparative study of metal removal efficiency. |
| 1.2 Crude (Untreated flame of forest seeds): Flame of forest seeds were sun dried, powdered, sieved using 60/72 mesh BSS Standard sieve to get uniform sized particles. Powder so obtained was dried in the hot air oven for 2 hours at 60 ºC. |
| 1.3 Preparation of activated carbon by pyrolysis: The activated carbon used in this study was prepared by complete pyrolysis by carbonizing flame of forest seeds in a muffle furnace in the absence of air. The crude powder was pyrolysed at a temperature of 500°C for 30 minutes. It was cooled in a desiccator and stored in air tight pouch. |
2. Preparation of chromium stock solution: |
| Potassium dichromate (K2Cr2O7) was dissolved in distilled water to prepare Chromium stock solution. 1000 ppm of stock chromium solution was prepared by dissolving 2.83 mg of potassium dichromate in one litre of double distilled water. Other needed concentrations were obtained by diluting the stock solution. The pH of the solution was maintained at the required value [5]. |
3. Preparation of Di Phenyl Carbazide (DPC) solution: |
| DiphenylCarbazide solution was prepared by dissolving 250mg of DPC in 50ml of acetone in a 100 ml volumetric flask [5]. |
4. Analysis of chromium: |
| 0.25ml of phosphoric acid was added to 1ml of standard sample containing known concentration of chromium, pH was adjusted to 1.0±0.3 using 0.2N sulphuric acid. The solution was mixed well and then diluted to 100 ml in a volumetric flask using double distilled water. Further 2ml of DPC solution was added and mixed well. After full colour development for 10 min, 4ml of this solution was used in absorption cell and the absorbance were measured spectrometrically at 540nm in UV-double beam spectrophotometer [5]. The absorbance of different known concentrations of chromium solutions was determined and a graph between concentrations versus absorbance was plotted. A straight line was obtained with R2 of 0.995. |
5. Batch Experiments |
| Batch adsorption studies were performed by shaking 100 ml of solutions in 250 ml conical flasks fitted with cork lid kept in an orbital shaker [6]. Experiments were conducted by varying one of the parameter and keeping other parameters at constant values. The adsorptions of Cr ions were computed from the change in metal concentration in the aqueous solution before and after equilibrium by using the following equation. |
| Where qe is adsorbed metal (mg/g adsorbent), V is the volume of the solution (l), W is the amount of adsorbent (g), and Co and Ce (mg/l) are the initial and equilibrium chromium concentrations of the solution respectively. The chromium percent removal was calculated using the following equation: |
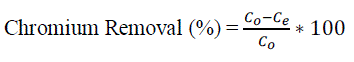 |
III. RESULTS AND DISCUSSION |
1.Effect of pH: |
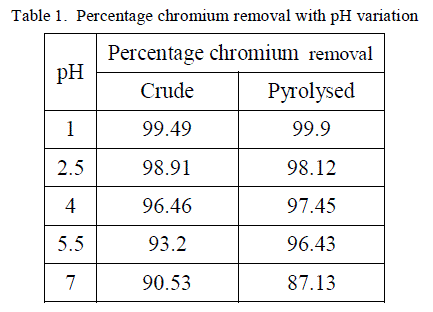
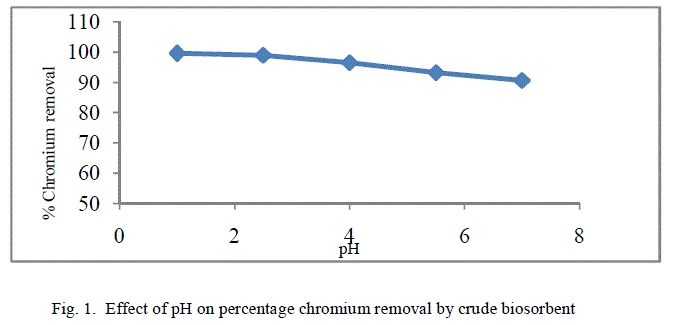
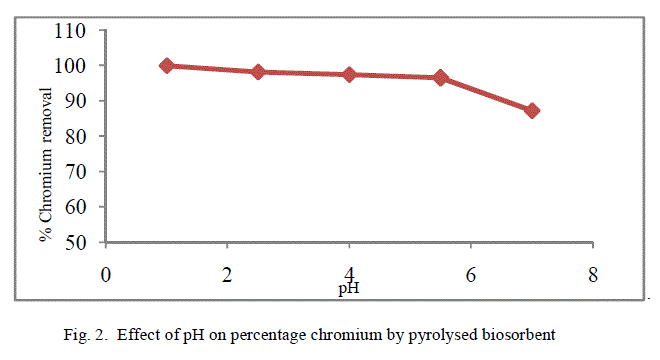
| The removal of poisonous heavy metals from waste water by biosorption very much depends on pH of the solution which impacts the surface rate of the adsorbent and the level of ionisation and specification of adsorbate. Table 1 presents the percentage chromium removal for different values of pH. Figure 1 and 2 shows the variation of percentage chromium removal with pH for crude adsorbent and pyrolysed adsorbent respectively. It was observed that high percentage chromium removal was observed at low pH of about 1. This may be attributed to fact that in acidic pH the adsorbent surface may be protonated and hence positively charged adsorbent removes higher amounts of chromium in the anionic form HCrO4 -. With increase in the pH of the system the level of protonation of the surface reduced step by step thus lowering the percentage chromium removal. |
 |
 |
 |
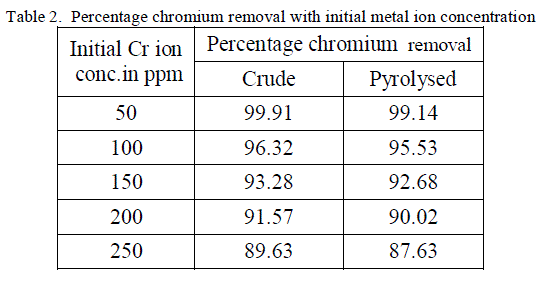
2. Effect of Initial metal ion concentration |
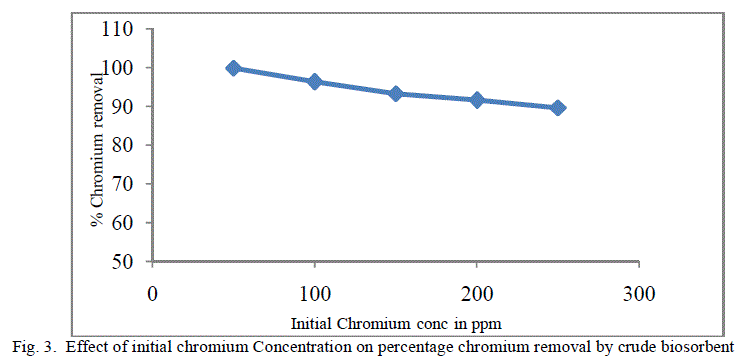
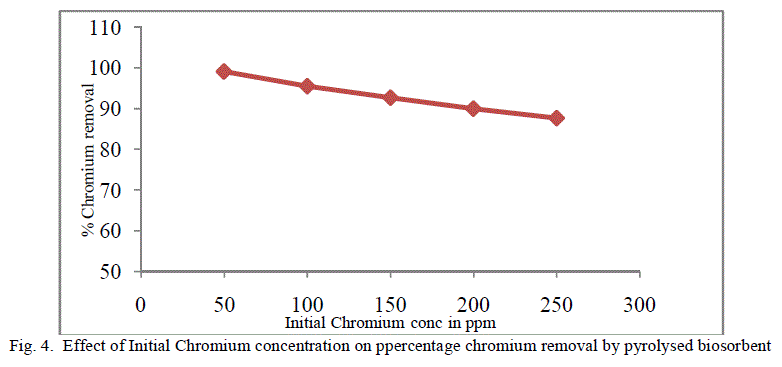
| The equilibrium time needed for the biosorption of chromium with two forms of flame of forest seeds was studied varying initial concentrations from 50-250 mg/l keeping other conditions at constant values. Figure 3 and 4 represents the effect of initial concentration on percentage chromium removal by crude and pyrolysed adsorbent respectively. As expected, adsorption capacity decreases with increase in initial ion concentration. It can be inferred from the Figures 3 and 4, that maximum removal is achieved at the initial concentration of 50 to 100 ppm. This is due to the fact that at lower initial concentration adequate adsorption sites are accessible for adsorption of chromium ions and at higher concentrations the chromium ions will be more than the available adsorption sites. |
 |
 |
 |
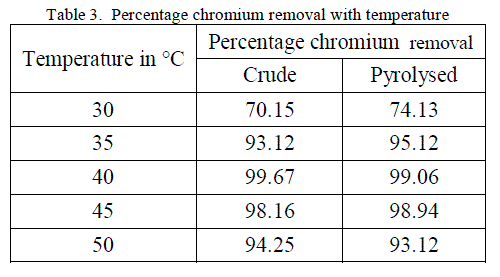
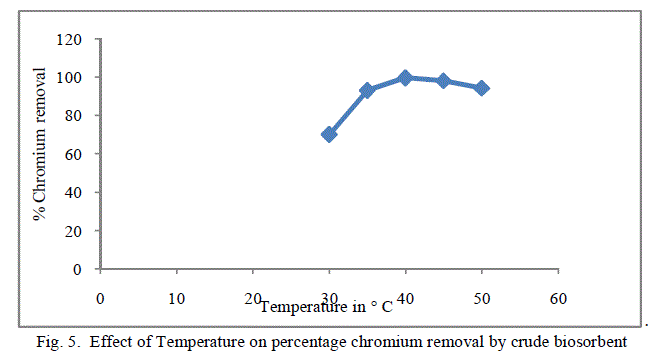
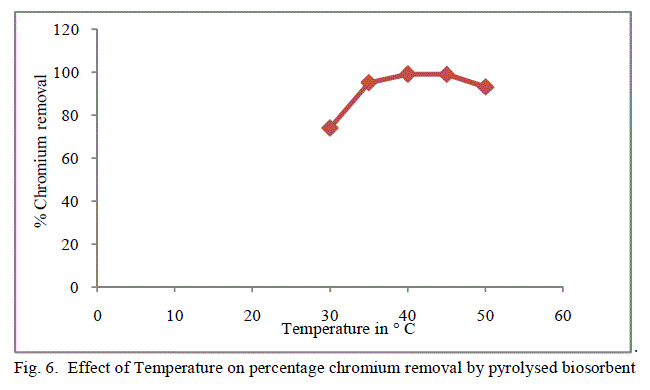
3. Effect of Temperature |
| Temperature effect on the biosorption of chromium was studied by varying the temperature between 30oC to 50oC for both forms of flame of forest seeds. Table 3, Figure 5 and 6 demonstrates the effect of temperature on percentage chromium removal by crude and pyrolysed adsorbent respectively. A slight increase in adsorption is observed from 30oC to 40oC and there is a decrease in percentage removal of chromium with increase in temperature. This behaviour is due to the slight exothermic behaviour of adsorption process. |
 |
 |
 |
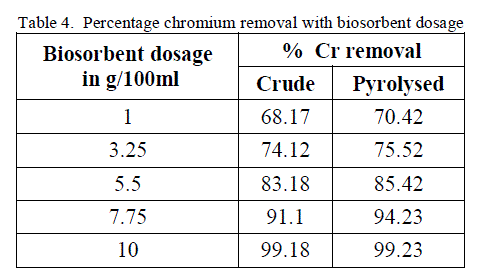
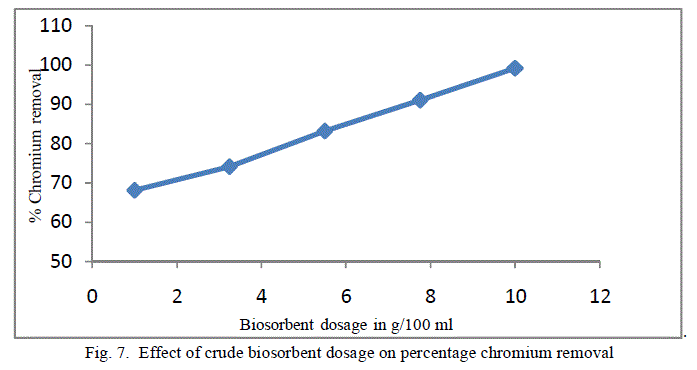
4. Effect of Biosorbent dosage |
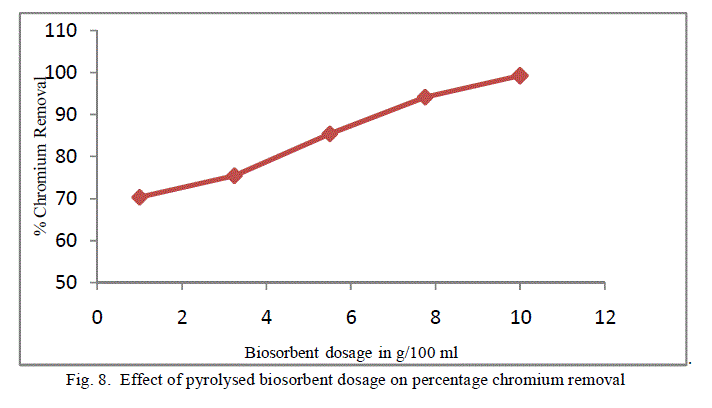
| To analyse the impact of biosorbent dosage on the chromium removal, biomass loading is varied from 1 g/100ml to 10 g/100 ml keeping other conditions at constant values. The obtained results are shown in figure 7 and 8. There is an increase in removal of chromium ion with increase of biosorbent dosage as exhibited by both the adsorbent. It is apparent that the chromium ion removal increases with increase in adsorbent dosage due to the higher availability of the replaceable active sites or the surface area for adsorption. Moreover the percentage of metal ion adsorption on adsorbent is confirmed by adsorption capacity of the adsorbent. |
 |
 |
 |
IV. CONCLUSIONS |
| Studies on Biosorption of hexavalent Chromium was carried out by evaluating percentage removal at different conditions and considering initial metal ion concentration, pH, temperature and biomass loading as variables affecting the process. Crude flame of forest seeds and pyrolysed flame of forest seeds were employed for the study. The parameters considered for the study were varied in the particular range. High chromium removal is possible at low pH of about 1, high adsorbent dosage and low initial concentrations. Further, highest chromium removal was possible at moderate temperature of 400C. |
References |
|