e-ISSN: 2319-9849
e-ISSN: 2319-9849
Anna Sears*, Pavel Kus, Semen Gogulin, Sara Doubravska
Department of Chemical Engineering, Research Centre Rez, Husinec Rez, Czech Republic, Europe
Received: 16-Nov-2022, Manuscript No. JCHEM-22-80074; Editor assigned: 18-Nov-2022, Pre QC No. JCHEM-22-80074 (PQ); Reviewed: 02-Dec-2022, QC No. JCHEM-22-80074; Revised: 09-Dec-2022, Manuscript No. JCHEM-22-80074(R); Published: 16-Dec-2022, DOI: 10.4172/2319-9849.11.7.002.
Visit for more related articles at Research & Reviews: Journal of Chemistry
Ultrafiltration is a membrane process used in various industries, from drinking to wastewater treatment. In nuclear research and energy, ultrafiltration removes actinoids in colloidal or pseudo-colloidal form from wastewater. The study aimed to determine the possible use of ultrafiltration to remove dissolved metals, specifically iron and aluminium, from a solution using polyacrylic acid as an organic polymer. Three solutions were tested containing the metals of interest and boric acid, which is standardly present in the liquid nuclear waste at the LVR-15 reactor. The results showed that a well-chosen combination of ultrafiltration membrane, pH and organic polymer Al3+ and Fe3+ could be successfully removed from the solution.
Polymer-assisted ultrafiltration; Membrane technologies; Metal removal; Liquid radioactive waste; Organic polymer
Various types of membrane separation processes have been developed in the past decades. These processes found their application in the production of drinking water and later in the purification of various processes and wastewater. The expansion of the use of membrane technologies has become the reason for creating a whole range of commercial products, whether they are membranes or complex technological solutions. Due to the sufficient durability of membranes and their proven functionality in conventional water treatment technologies, membrane technologies are an alternative for processing liquid radioactive waste in addition to traditional technologies, such as sorption in the nuclear industry. This does not necessarily mean a complete replacement of conventional technologies, but membranes can provide support in cases where traditional technologies are not efficient or economical enough.
Ultrafiltration is used in a wide range of industries, from drinking water and wastewater treatment to the chemical, food and pharmaceutical industries, to recycle the flow or add value to downstream products. In the nuclear sector, ultrafiltration is often used to remove alpha radioactivity from wastewater. Actinoids contained in wastewater are often present in colloidal or pseudo-colloidal forms and can be effectively removed by ultrafiltration. In addition to removing colloidal particles from solutions, ultrafiltration can be used as a pre-treatment step to reverse osmosis [1].
Several studies about the treatment of radioactive waste using ultrafiltration have been published. In most cases, ultrafiltration happens after the addition of a chemical substance. These methods use surface-active substances, so-called Micellar Enhanced Ultrafiltration (MEUF) or polymerisation agents in the case of seeded ultrafiltration/Polymer-Assisted Ultrafiltration (PAUF). In the case of the first variation, the use of Sodium Dodecyl Sulphate (SDS) as a surface-active substance was described [2]. SDS was added to the processed solution, and after several hours of mixing, this solution was subsequently purified through a membrane. The highest retention of radioactive metals was achieved in pH 8-11 when most radioactive metals apart from Cs ions were almost completely removed.
In the case of PAUF, which involved the use of reagents such as Polyethyleneimine (PEI) and Microcrystalline Chitosan (MCH) for radionuclides Co-60 and Cr-51 [3]. The process is similar to MEUF–addition of a reagent, mixing, reaction and subsequent filtration through a membrane. The decontamination factors were 52 to 68 for the laboratory apparatus and 37 to 62 for the pilot unit, respectively. In addition to PEI, Polyacrylic Acid (PAA) to remove Co-60 and Sr-90 through a regenerated cellulose membrane was described [4]. Retention reached values comparable to low-pressure RO. The use of PEI was also tested for the removal of Cu, Ni and Zn in combination with a cellulose acetate membrane [5]. Metal retention increased with increasing pH and decreased slightly in the presence of salts (NaCl, K2SO4). The above reagents did not significantly remove Cs-137. Copper Hexacyanoferrate (CuFC) at alkaline pH and Nickel Hexacyanoferrate (NiFC) in combination with a composite ultrafiltration membrane were used to remove Cs-137 from the solution [3,6]. An increase in Cs retention by the addition of SDS together with NiFC. The tests in the study took place with real liquid Radioactive Waste (RAW) [7]. The solution contained Co-60, Zn-65, Ba-133, Cs-134, Cs-137, Eu-152 and Am-241. Ultrafiltration was carried out through a ceramic membrane, and the reagents used were sodium salt of Polyacrylic Acid (NaPAA), reagent INSTAR AS (copolymer of macromolecular acrylamide and Sodium Polyacrylate) and Cobalt Hexacyanoferrate (CoCF). RAW processing was carried out similarly as in the above examples. According to the study, the best results were achieved with a combination of all three listed agents. In experiments with a real RAW solution were carried out, ultrafiltration was performed using a ceramic membrane and NaPAA and CoCF reagents [8]. This study found a reduction in decontamination factors when both agents were added simultaneously. A two-phase system was proposed, In the first phase, CoCF will be added, followed by filtration through the membrane. In the second phase, NaPAA will be added to the obtained permeate and filtrate again through the membrane.
The research infrastructure of the Research Center Rez, the LVR-15 reactor, produces various types of liquid waste (different compositions of radionuclides, the content of organic substances, etc.). The spectrum of waste ranges from pure water to saline solutions containing boric acid, iron, and aluminium. Water purification is complicated because it contains radionuclides, making it difficult to manage these wastes. For this reason, it is ideal to use ultrafiltration, during which all unwanted substances are concentrated in one stream and purified water in the other stream (in the case of crossflow) or simple filtration with occasional flushing of the membrane (in the case of dead-end). This work aimed to test the possibility of using a PAUF to remove iron and aluminium ions from the simulated liquid radioactive waste.
Chemicals
Non-active aqueous solution simulating feed waste contained boric acid (1 g/l), Fe3+ (1 mg/l) as ferric nitrate nonahydrate (Fisher Chemical) and Al3+ (1 mg/l) as aluminium nitrate nonahydrate (Fisher Chemical). Potassium hydroxide (Lachner) was used to adjust the pH, and Polyacrylic Acid PAA (Sigma Aldrich) with a molecular weight (Mw) of 100 000 and 250 000 was used as an organic polymer. All chemicals were in analytical grade. A set amount of PAA was added to the tested solution to obtain a PAA to M3+ ratio ranging between 5 to 50.
Ultrafiltration system
The experiments were performed using an ultrafiltration laboratory unit for testing flat sheet membranes consisting of a high-pressure pump and a filtration chamber (Figure 1). After the addition of PAA, the tested solution was stirred for 30 minutes at a stirring speed of 100 rpm using a magnetic stirrer (IKA) and then left to react for another 60 minutes. A pre-dried and weighed polysulfone membrane GHPS Media 0.02 A Micron 47 MM disc (Filtration group) was used. The flow rate through the unit was set to 20 ml/min, and the operating temperature was 22 ± 2ºC. Three solutions were tested; one consisted of boric acid and Fe3+, the second with boric acid and Al3+ and the last with boric acid and both trivalent metals. Each experimental solution was tested at both alkaline and acidic pH. Feed waste solution and permeate samples were collected to analyse the metal content, pH and conductivity.
Analysis
The concentration of the metal ions in the feed water and permeate was measured using a ContrAA 700 High-resolution continuum source atomic absorption spectrometer (Analytik Jena, Germany), UV/Vis Spectrophotometer Jenway 6850 (Cole-Parmer Instrument Company, USA) and a WTW Series Photolab S12 (Xylem Analytics, Germany).
The conductivity and pH values in all solutions were measured using a WTW pH/Cond 3320 (Xylem Analytics, Germany).
The removal efficiency of each metal was calculated as follows:
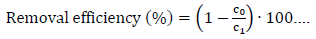
Where c0 is the concentration of metal ions in the feed waste solution and c1 is the concentration of metal ions in the permeate.
Removal of ferric ions
The influence of an added organic polymer on metal removal efficiency using an ultrafiltration membrane was investigated first with the solution containing boric acid and Fe3+. The removal efficiency using PAA of Mw 100 000 at alkaline pH increased with the PAA: Fe3+ ratio. A significant rejection of Fe3+ higher than 90% occurred at the ratios from 20 to 50. In the case of PAA Mw 250 000 at the alkaline condition, an opposite trend was observed. Higher Fe3+ rejection efficiency of up to 95% was achieved with a lower PAA: Fe3+ ratio (10 and 20). A decreasing rejection efficiency trend was observed with a higher PAA: Fe3+ ratio. In the acidic environment, low Fe3+ removal was achieved for both molecular weights of PAA compared to alkaline conditions. The rejection efficiency decreased in both cases of PAA Mw with an increasing PAA: Fe3+ ratio. The comparison of the removal efficiency is shown in Figure 2.
Removal of aluminium ions
Aluminium was removed by this method at acidic pH only at a lower organic polymer-to-metal ratio, namely 12% and 36% when using PAA Mw 100 000 and Mw 250 000, respectively. As the ratio increased, Al3+ was no longer removed from the solution. A similar situation occurred with PAA Mw 250 000 additions in an alkaline environment where the efficiency of removing Al3+ decreased with increasing PAA: Al3+ ratio from 14% to zero values. When PAA Mw 100 000 was used, the decrease in ion removal efficiency was not so significant. Still, the highest values were at 18% and decreased with increasing PAA to metal ratio to the value of 7%. The metal removal efficiency as a function of the PAA: Al3+ ratio in the PAUF system in both acidic and alkaline conditions is shown in Figure 3.
Removal of both ferric and aluminium ions
A solution with Fe3+, Al3+ and boric acid was tested to investigate the possibility of simultaneous removal of both metals with the PAUF system using a PAA of Mw 100 000. The comparison of the removal efficiencies of both trivalent metal ions from the solution is shown in Figure 4. A decreased rejection with an increasing PAA: M3+ ratio of both metals was observed at pH 4. The removal efficiencies were 94% for Fe3+ and 74% for Al3+ at the PAA: M3+ ratio of 2.5 and decreased with increasing ratios to 35% and zero, respectively. This corresponds with the results of the individual metal testing at acidic pH. Nevertheless, it can be stated that the presence of Fe3+ in the solution positively affected Al3+ rejection at pH 4. The removal efficiency was 9% when the Fe3+ was not present in the solution compared to 74% when it was. The same effect had the presence of Al3+ on the removal efficiency of Fe3+, which was at its highest of 45% without the presence of Al3+ compared to 94% with Al3+ in the solution. At pH 8, the Fe3+ removal efficiency increased from zero values to 35%, significantly lower than when no other metal was present in the solution. In this test, the Al3+ was not removed from the solution.
Simultaneous removal of both metal ions was tested with PAA of Mw 250 000 to compare the effect of different molecular weights of organic polymer. In the acidic environment, the removal efficiency of Fe3+ and Al3+ from the solution reached a maximum value of 96% and 89%, respectively, at a lower PAA: M3+ ratio. The values then decreased with more PAA added to the solution. Comparing the molecular weights of PAA, the results were similar to those using Mw 100 000, where the removal efficiencies were slightly lower than when using Mw 250 000. The presence of Fe3+ enhanced the rejection of Al3+ from 37% in a single metal solution to 89% at the lower PAA: M3+ ratio. Correspondingly, the presence of Al3+ in the solution positively affected Fe3+ removal at pH 4. The highest value of removal efficiency was 16% without the presence of Al3+ compared to 96% with it.
In the alkaline environment, high rejection of Fe3+ (90%) occurred at the ratios from 5 and 10 and then decreased. The overall removal efficiency of Fe3+ was slightly lower in the presence of another metal. On the contrary, like previous tests, the presence of Fe3+ positively influenced the removal of Al3+ at 58% compared to 14%. The trend of the removal efficiency of Fe3+ and Al3+ from the solution using the combination of ultrafiltration and PAA at different PAA: M3+ ratios and different pH is shown in Figure 5.
The influence of an added organic polymer in the PAUF system on metal removal using a polysulfone flat-sheet ultrafiltration membrane was investigated. Three solutions were tested, one with Fe3+, the second with Al3+ and the third with both. Boric acid was present in all solutions and PAA was used as an organic polymer in two different molecular weights. The results with the simulated wastewater indicate that a well-chosen combination of ultrafiltration membrane, a dose of organic polymer and pH, Al3+ and Fe3+, can be successfully removed from the boric acid solution.
The Fe3+ had an overall higher removal efficiency compared to Al3+. The removal rate of Fe3+ reached 95% in the alkaline environment for both tested molecular weights of PAA. In contrast, this method only removed Al3+ from the single metal solution to a limited extent, with a maximum removal efficiency of 37% at pH 4. Comparing the effect of pH on the single metal solution, the removal efficiency was generally higher in alkaline conditions for Fe3+. However, the simultaneous removal of both Fe3+ and Al3+ from the solution was more successful at pH 4 for both metals. In most cases, a negative influence of a higher PAA: M3+ ratio on the removal efficiency was observed. Therefore, it is essential to carefully determine the amount of PAA used for the best results.
It can be stated that the presence of Fe3+ in the solution had a positive effect on Al3+ rejection at pH 4 in the case of both types of PAA. Considering the influence of molecular weights of used PAA, the results were similar in the mixed metal solution, where the removal efficiencies were slightly lower using PAA Mw 100 000 than when using Mw 250 000.
The presented results were realised with the Institutional Support of the Ministry of Industry and Trade.
[Crossref] [Google scholar] [EBSCO]
[Crossref] [Google scholar] [EBSCO]
[Crossref] [Google scholar] [EBSCO]
[Crossref] [Google scholar] [EBSCO]
[Crossref] [Google scholar] [EBSCO]
[Crossref] [Google scholar] [EBSCO]