ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Prof.S.C.Borse, Y.E.Mangulkar
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In advanced countries, researches related to the machinability & microstructures of gray cast iron were carried out early, especially on the effect of inoculants. Also there has been an increasing need and demand for the processes to obtain carbide free, thin section grey iron castings. Inoculation is a well established process which not only prevents carbide formation, but also controls the size and form of graphite flake in the grey cast iron. This paper reviews some of the developments that have taken place in the inoculants and inoculating procedure grey cast iron. Also some of the more recent inoculants which have been used by various researchers with various degree successes have been briefly explained.
Keywords |
| Eutectic cells, Grey cast iron, Inoculation, Nucleation. |
INTRODUCTION |
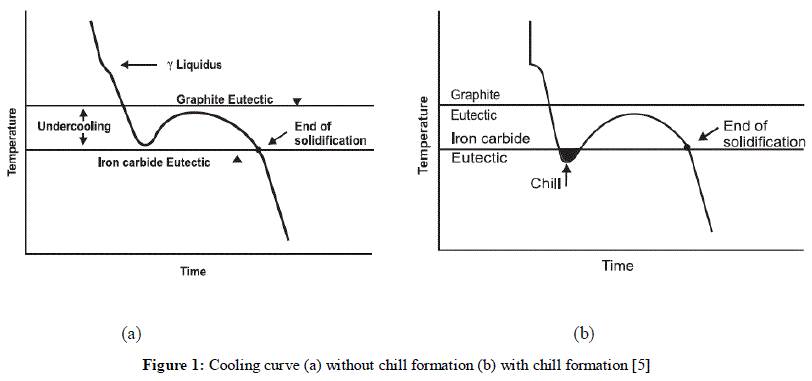
| Inoculation is nowadays a commonly applied metallurgical treatment carried out by foundries to improve the mechanical properties of commercial alloys. The essence of the cast iron inoculation consists in changing the physicochemical state of molten metal. It is a mean of controlling the structure and properties of cast irons by increasing the number of nucleation sites available for the growth of graphite flakes in grey irons or graphite nodules in ductile irons. The change is obtained by introducing to the cast iron of low graphite nucleation power, shortly before mould pouring, a small amount of inoculant, that is, of a compound capable of increasing the number of active nuclei. The main criterion used in evaluation of the inoculation effects are changes in the microstructure, mechanical properties of cast iron along with its chilling tendency. The solidification of hypoeutectic cast iron is a nucleation and growth process that occurs in stages. When a melt has cooled to the liquidus temperature solidification begins with the nucleation and subsequent growth of austenitic dendrites. As the temperature continues to decrease, the remaining liquid becomes enriched in carbon until the eutectic composition is attained at the eutectic temperature. The melt continues to cool and when sufficiently undercooled the austenite-graphite eutectic transformation begins. The transformation from liquid to solid is accomplished by nucleation at a number of discrete locations (nuclei) called eutectic cells and proceeds by the growth of these cells along a spheroidal crystallization path. The eutectic cells continue to grow until they have impinged on one another and solidification is essentially complete. The form and distribution of the graphite phase of the eutectic is determined by the composition, cooling rate, and degree of nucleation of the melt.[1] Inoculation is a mean of controlling the structure and properties of cast iron by minimizing undercooling and increasing the number of nucleation sites during solidification. This means to prevent the undercooling to temperatures below the metastable eutectic where carbidic structures are formed (Figure 1). The iron solidification mechanism is prone to form chilled iron structures when inoculation is inadequate. Chilled structures interfere with the machining, necessitate additional heat treatment operations, results in non-conformance with specifications and, in general, increase the total cost of production. [2] |
 |
II.HISTORY OF INOCULATION |
| In 75 years, the cast iron industry has seen major changes in techniques and methods of manufacture and in the understanding of its basic metallurgy. Inoculation has a vital role to play in the continuing progress of cast iron.The search for high strength cast irons was based on the knowledge that the reduction of carbon and silicon to lower levels was essential for high strength development. Steel additions were made to the cupola charge from about the time of the outbreak of the war, and the confusing term "semi-steel" was coined for any cast iron where more than about 15 % of steel scrap had been included in the charge. The term remained in vogue for some 30-40 years before its eventual disappearance. In the early days, poor control over the cupola and the iron analysis resulted in many disappointments, and even in the 1930's many foundry managers would still not tolerate steel in the cupola. Processes were developed which produced cast irons with special properties by using heated or chill moulds, jolting or shaking the iron, or using special charges. Although they flourished for a while, they just as quickly faded away. All of these processes were in fact German, but it was in the USA and in Great Britain in the early 1920s that inoculation began to emerge, although not yet so called. [3] In the early years of the 1920s, a number of foundry men investigated the effects of ladle additions on cast irons. Some of this work was done in secret, some of it was published, and some of it formed the basis of a variety of patents. Silicon control was in the forefront of everyone's minds. Strong iron was dependant on obtaining the lowest possible silicon content consistent with a grey fracture and machineability of thin sections. Crosby at the Studebaker Foundry added graphite and ferrosilicon (1922-1923), Meehan added calcium silicide and magnesium silicide (1922), Smalley used ferrosilicon, calcium silicide, and zirconium silicon (1922-1924), and Moldenke added aluminum (1921). The objective of all these various additions to the ladle were to control the graphite size and shape in low carbon equivalent iron, to promote A-type flakes instead of fine under-cooled forms, to obtain freedom from chill in thin sections, to promote uniformity throughout different section sizes, and to improve machineability. These are exactly the same objectives for which inoculants are still used today. [3] |
| In the 1920s, the process was seen as one of scavenging, refining, and deoxidizing the melt, and it was not yet understood as one of nucleation. The metallurgy of the solidification of cast irons was as yet imperfectly understood, and much argument raged over whether graphite solidified directly from the melt, or whether it was always a decomposition product of primary solidification of cementite. For instance, the Meehanite Corporation specifications showing the use of ferrosilicon or calcium silicide additions first appeared in the U.K. in 1928. There was no mention of the word inoculation in the 1920s, and the treatments were simply referred to as ladle additions. The terms inoculation and inoculants belong to the next period in history, and their originator is unknown. Ferrosilicon containing 75-80% silicon became established as the most common inoculant. The control of calcium and aluminum in ferrosilicon had been far from satisfactory in the early days, which probably helped the initial success of calcium silicide. It was not until 1938 that Lorig Kinnear and Barlow suggested that calcium and aluminum were the vital elements in ferrosilicon, and it was left to McLure et.al (1957) and Dawson (1961) to indicate the optimum contents. Graphite itself was proved to be a valuable inoculant by both Norbury and Morgan and Massari and Linsay. The magnesium method pioneered by International Nickel and the cerium method of Morrogh and Williams were both announced at a historic AFS Congress in Philadelphia in 1948. Because both magnesium and cerium are carbide stabilizers and the manufacturing of ductile iron requires an inoculation step, so inoculation received another boost. Ferrosilicon rapidly became the almost universal product to be applied in ductile iron.In grey iron the 1940s was a time of considering the best methods of adding the existing selection of inoculants. It was soon proved that the most effective method was into the stream of metal entering the ladle, and a number of simple devices were invented to do this. [3] However, further modifications to inoculation theories were proposed, e.g. the solidification of undercooled graphite was finally proved to be just as much a product of solidification from the liquid during the eutectic reaction as are random flakes by Hurum in 1952. The most notable suggestion for a new inoculant was the use of barium in a silicon-manganese alloy base by Kessler in 1956. Commercial barium-containing inoculants were developed by the Vanadium Corporation and Baranov, and strontium ferrosilicon was prepared by BCIRA and Union Carbide after the work of Dawson and Clark and McCluhan. Cerium-bearing inoculants were proposed, and appeared from the Vanadium Corporation. Mixed inoculants giving good control over the very potent effects of graphite were introduced by Foseco. The problem of fading had been recognized very early on, and it was acknowledged that inoculated metal had to be poured in 10 minutes or less to avoid this. In the mid-1960s, the problem was tackled in a different way, and inoculation of the metal directly in the mould was attempted with no further problem in fading. The investigations of Dell and Crist, Trager and Kaune, Hall, Bakkerus and Gaertman, and Ryzhikov proved that the concept was viable by using a variety of inoculants either as fines, lumps, pellets, or tablets. Karsay used a different technique and inoculated in the spout of the ladle with a tube filled with inoculant. [3] |
III.LITRATURE SURVEY |
| J.N. Harvey Et.Al Described The Necessity To Inoculate The Cast Iron And The Differences In Inoculation Techniques Of Grey And Ductile Cast Iron. He Also Explored The Effects Of Various Inoculant Materials On The Microstructure Of Cast Iron. Also, Case Studies Based On Changes In Inoculation Methods, Inoculants And Cast Iron Grade.[3] N.T. Skjegstad Et.Al Described Important Conditions In The Production Of Cast Iron Which Call For The Addition Of An Inoculant To Ensure The Reliable Production Of Sound, Strong, And Machinable Castings. The Principal Differences Between Inoculated And Un-Inoculated Cast Irons Are Described, And These Differences Are Exemplified By Characteristic Microstructures And Mechanical Properties. Also, Some Practical Considerations Related To Various Methods Of Inoculant Addition To Liquid Iron Are Explained. [4] J.O. Choi Et.Al Investigated That Effect Of Rare Earth Metal(RE) On Microstructural Features In Thin Wall Ductile Iron Castings, Including The Thickness Of Ferrite Layer Around Graphite Ferrite, Graphite Nodule Size, And Graphite Nodule Count, Depend On The Amount Of Rare Earth Elements And Sample Thickness. The Addition Of RE Leads To A Decrease In The Amount Of Chill Formation, A Higher Graphite Nodule Count And Size As Compared To Those In The Specimens Without RE. He Suggested That The Role Of RE Varies With Sample Thickness. Irrespective Of The Addition Of RE, Suggesting That The Effect Of Rare Earth In Reduces Chill Formation In Very Thin Sections. Due To The RE Addition Nodularity Of Graphite Nodules Improved, A Lower Tensile And Yield Strength Reduced In Specimens Without RE. [5] |
| S.O. Olsen Et.Al Described About Important Conditions In The Production Of Cast Iron And Characteristic Microstructures And Mechanical Properties Exemplify The Difference Between Inoculated And Un-Inoculated Irons. Principal Mechanisms Of Inoculation And Graphite Nucleation In Grey And Ductile Irons Are Described. The Findings Are Based On Advanced Electron Microscopy Studies Of Micro-Particles As Heterogeneous Nucleation Sites For Graphite. Effects Of Minor Alloying Elements Such As Ca, Ba, Sr, And Al Are Explained As Well As The Critical Role Of Oxygen And Sulphur In The Graphite Nucleation Process. [6] Iulian Riposan Et Al. Investigated The Effect Of Strong Deoxidizing Elements, Such As Al, Zr, And Ti, In Gray Irons, With The Same 0.03 Wt. % Addition. Al And Zr Have Visible Beneficial Effects, By Lowering The Degree Of Eutectic Undercooling, Chilling Tendency, Undercooled Graphite, And Free Carbides Amount, In Both Uninoculated And Inoculated Irons. Ti Seems To Be Beneficial As Graphitizing Action Only In Un-Inoculated Irons, But At Lower Relative Power Compared To Al And Zr. It Was Re-Confirmed That Complex (Mn,X)S Compounds Nucleated On The Previously Formed Very Small Oxide Based Sites Act As Major Nucleation Sites For Graphite Flakes With Specific Distribution Of Al, Ti, And Zr.[7] V.Popovski, Et.Al Studied Influence Of Ledeburitic Cementite On The Castings Of Unacceptable Quality And The Effect Of Inoculation Of The Complex Inoculants Based On Barium Produced By Two Different Suppliers. The Research Showed That The Quantity Of The Added Inoculant Has An Effect On The Formation Of Ledeburitic Cementite Which Impairs The Mechanical Machinability. Inoculants Help To Balance The Hardness On The Castings. The Measurements Showed That The Difference In Hardness Of The Castings At Different Wall Thicknesses Is Smaller In Cases, Where Inoculants With Less Barium Were Used And They Also Were Alloyed With Calcium And Also Contained No Aluminium. Occurance Of Ledeburitic Cementite Is Undesired Because Such Castings Cannot Be Machined With Removing Material. Thin Walled Castings Are Especially Sensitive To Precipitation Of Cementite. The Observed Casting Had The Wall Thickness Of 6 Mm To 7 Mm On The Base And 25 Mm In The Center. Hardness Measurements And Microscopic Test Showed That The Most Suitable Inoculant For The Chosen Casting Was The One That Besided Iron And Silicon Contains Also 2 % To 2.5 % Of Barium And 2 % To 2.7 % Of Calcium. [8] |
IV. METHODS OF INOCULATION |
A. Preconditioning |
| Preconditioning describes the addition of a particular inoculant to the clean, deslagged surface of a furnace melt prior to the nodulizing treatment process. This event serves to normalize the nucleation potential of the melt. The inoculant may be high purity graphite, inoculating grade ferrosilicon, or silicon carbide grain. In each case, the addition must be of a size that will dissolve quickly. This is particularly true for silicon carbide as this addition dissolves rather than melts. Typically, an addition of 0.1% or 1 kg per metric ton (2 lb per US ton) is sufficient to enhance the formation of nuclei.[9] |
B. Pre-inoculation |
| At this stage, an addition of inoculating grade ferrosilicon is made either to the stream of iron filling the treatment ladle, or as part of the alloy “package” in the treatment ladle. The inoculant may be part of the cover material in a sandwich or tundish treatment. Combining the Mg alloy and the ferrosilicon inoculant can contribute to weaken the carbide promoting effect of Mg and to adjust the silicon content in the iron according to the grade targeted and the casting section size. As in the other inoculation steps, the primary function of the inoculant is to add nucleation sites to facilitate the growth of graphite particles versus the growth of carbides.[9] |
C. Ladle Inoculation |
| This is the classic inoculation method, the inoculant being added during tapping or pouring, e.g. after a magnesium treatment. Depending on the amounts of iron, inoculants with grain sizes between 0.6 mm and 6 mm are mostly used for this type of inoculation. It must be ensured that the inoculant is not placed on the bottom of the ladle but added as steady as possible to the iron stream. Ladle inoculation is often referred to as “post inoculation” is possibly the most important step of the inoculation procedure. Because of the violence of the Mg reaction during the spheroidization treatment, a significant fraction of the nuclei generated by steps 1 and 2 can become entrained in the surface slag covering the iron melt. Magnesium treated melts also tend toward more undercooling. It is therefore necessary to rehabilitate the nucleation potential of the iron. [9] |
D. Late Inoculation |
| Late inoculation is however the most effective step in the inoculation series. It is well known that the effects of both the magnesium treatment process as well as the addition of inoculants fade with time. By moving the inoculation event to as late in the pouring process as possible, the effects of fading are minimized. Often, a small addition of a late inoculant can replace a much larger addition at earlier steps. In some cases, late inoculation may be avoidable. This last step can serve as safety procedure rather than an absolutely required one. In some instances, late inoculation compensates for less than optimal charge composition and/or melting procedure and/or spheroidization processes. For example, although late inoculation does not completely offset the effect of deleterious elements such as Cr, V, Mo, Sb, V& Ti it can minimize the detrimental effect by increasing nodule count and decreasing the degree of segregation.[9] |
| Control of the inoculation practice is very important, and the correct technique must be used to obtain satisfactory and consistent results. Inoculants must be well mixed with the molten iron to obtain uniform and complete solution. Good mixing can be obtained by either adding to the tapping stream from the furnace or when transferring from ladle to ladle. The practice of placing the inoculant in the bottom of the ladle before starting to fill is not recommended. [2] Factors influencing the choice of inoculating method are [7]: 1. The time from filling the ladle to pouring the last casting, commonly known as the fade time. 2. Metal temperature. 3. Ability to add the inoculant at a particular point in the process. 4. Suitability of the casting system to late stream inoculation. Inoculants are generally added to cast irons at one or more of four stages during the casting procedure as shown in figure 2 [2],[7]: 1. To the transfer ladle. 2. To the pouring ladle. 3. To the stream of metal as it enters the mould. 4. Using an insert placed inside the mould runner system |
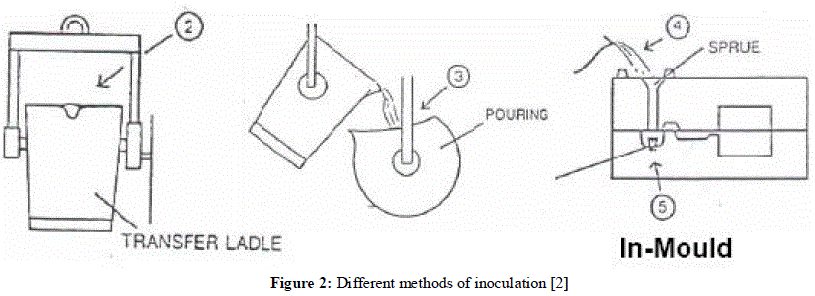 |
| Use of an insert made from pressed or cast inoculant is rarely used as the primary source of inoculation. Different size and composition tablets are available and prove particularly valuable when the fade time is long, acting as a secondary inoculation, only when late metal stream treatment is not possible. The possibility of human error in failing to add the tablet to a mould does necessitate a high degree of post casting inspection, usually in the few cases where tablets are used as the only inoculant. [7] If long holding times after inoculation cannot be avoided, inoculation may be topped up with an extra, small addition. Alternatively, some form of late inoculation may be considered. Late metal stream inoculation virtually eliminates fade. An inoculation method after the metal has left the ladle and enters the mould or within the mould itself, is referred to as late inoculation. Late inoculations, correctly applied, give the maximum effect obtainable from an inoculating addition, thus producing much higher levels of inoculation, and hence much lower chilling tendencies than by ladle inoculation alone. Late inoculation should be used when it is impossible to achieve and maintain adequate inoculation in the ladle. Late inoculation requires separate, extensive trials to establish a suitable process for each casting and, when established, the process must be strictly followed to avoid difficulties. Late inoculation may be used in addition to ladle inoculation in order to obtain an extra effect, but preliminary trials must first be made to ensure that it will be successful. [2] |
V.PRINCIPLES OF EFFECTIVE INOCULATION |
| 1. For effective inoculation a low base iron chill value should be maintained before treatment and inoculation. 2. Addition of the correct amount, type, and size of inoculants for the amount of metal to be treated and the casting section size/cooling rate. |
| 3. Excessive fines in the inoculant may leads to poor results of inoculation because they are already oxidized. 4. Over inoculation may leads to increase in the shrinkage tendency of metal. 5. High temperatures increase fading tendency and low temperatures promote carbide formation so care at time of addition of inoculant and pour metal should be the correct temperature for the casting section. 6. Proper mixing of the inoculant into the metal leads to homogeneous and fine microstructures. 7. Minimize oxidation of the metal after the inoculants has been added. 8. After completion of inoculation process the metal holding time should be as short as possible. [10] |
VI.INOCULANTS |
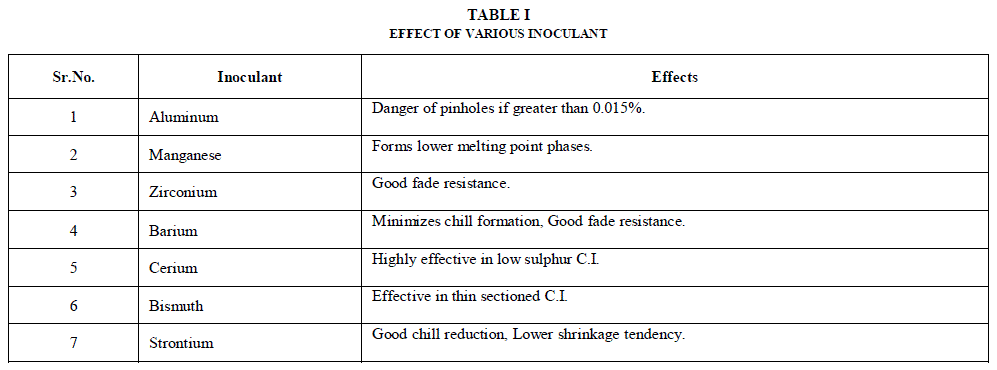
| An inoculant is a material added to the liquid iron just prior to casting that will provide a suitable phase for nucleation of graphite during the subsequent cooling. The purpose of inoculant is to assist in providing enough nucleation sites for the carbon to precipitate as graphite rather than iron carbide (cementite). Traditionally, inoculants have been based on graphite, ferrosilicon or calcium silicide. The most popular inoculant today is ferrosilicon containing small quantities of elements such as Al, Ba, Ca, Mn, Bi, Sr and Zr. [2] FeSi as a pure material has no inoculation effect. A combination of active elements e.g. Al, Ca, Ba, Mn, Zr, Sr, Bi when added to FeSi will inoculate. Amongst these; Ba, Zr, Sr, Bi are more powerful active elements than Al and Ca in FeSi based inoculants. This results in lower addition rates. [5] The sizing of the inoculant is usually ½ inches (13 mm) maximum. Since fines do not inoculate effectively, a minimum size limit of 1/6 inches (1.5 mm) is advisable. The inoculant should be stored in closed containers. Its effectiveness deteriorates with time when exposed to open air and moisture. [8] The prime effects of a few inoculants are listed in the table 1. |
 |
VII.EFFECT OF FADING |
A) Principal Effects of Fading |
| The effects of inoculation are at a maximum immediately after the addition of the inoculant. The rate of inoculant fading, which depends upon the composition of the inoculant and of the iron to which it is added, may be very rapid and much of the inoculation effect may be lost in the first few minutes after the addition. The principal effects of fading are [2]: 1) To cause greater undercooling to take place during eutectic solidification and to lead to a great tendency to chilling in grey and ductile irons, particularly in thin sections. 2) To reduce the number of eutectic cells growing in flake graphite irons resulting in a less uniform size distribution of graphite in the casting and a reduction in mechanical properties. |
| 3) To reduce the number of nodules formed in ductile iron and to cause deterioration in their shape. If sufficiently severe, the deterioration in shape may affect the mechanical properties of the castings. |
B) Facts about Fading |
| There are some well-established facts concerning fading which are of practical significance [2]: 1) All inoculants will fade after some time. 2) There is no period after inoculation during which fading does not occur. To obtain the maximum effect, metal should be cast as soon as possible after the addition of inoculant. 3) Some inoculants fade more slowly than others. 4) Inoculating effects vary according to inoculant composition. It is desirable that foundries should carry out tests to determine which the most suitable inoculant is for their specific purpose. |
VIII.CONCLUSION |
| From the above study it is observed that with variation of percentage in the inoculants and change in composition of base material we can improve the properties of base metal. So it is concluded that Inoculation is the one of the best suitable process to obtain the desired mechanical properties and microstructure of base metal by variation in the percentage of inoculants, variation in size of inoculants, temperature of base metal at the time of addition of inoculants and change in composition of the base metal. |
ACKNOWLEDGEMENT |
| The authors are really indebted towards the Director and Head of the Mechanical Engineering Department, Deogiri Institute of Engineering and Management Studies, Aurangabad. |
References |
|