ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ritu Paliwal1, Swatantra Singh2, Sanjay shukla2, Neelam Tandia2, Nitesh Kumar3 and Shailendra Singh2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Immunotoxicology is the study of immune dysfunction resulting from exposure of an organism to a xenobiotic. Immunotoxicology is relatively new interdisciplinary scientific field focused on identification and analysis of chemical and, in a broader sense, also physical and biological factors of the environment which can cause unwanted and usually incidental immunomodulations. The immune dysfunction may take the form of immunosuppression, allergy, autoimmunity and inflammatory-based diseases. Immune System plays a critical role in host resistance to disease as well as in normal homeostasis of an organism; identification of immunotoxic risk is significant in the protection of human, animal and wildlife health. In addition, immunotoxicology also investigates the properties of new immunotherapeutic pharmacological products prepared via recombinant DNA techniques (interleukins, interferons, growth factors, anti-inflammation drugs, neuroendocrine hormones, neuropeptides) with regard to their immunotoxic potential and safety of their use
Keywords |
| Immune system, Toxicity, human and animal health. |
I. INTRODUCTION |
| Immunotoxicology (ITOX) is the study of immune dysfunction resulting from exposure of an organism to a xenobiotic. The immune dysfunction may take the form of immunosuppression or alternatively, allergy, autoimmunity, or any number of inflammatory based diseases or pathologies. In the non-adult (embryo, fetus, neonate, juvenile, adolescent) this study is referred to as DEVELOPMENTAL IMMUNOTOXICOLOGY (DIT). For most toxicant examined to date, the developing immune system exhibits a heightened sensitivity compared with that of an adult. This reason, DIT screening has applications to human, animal and wildlife health protection. |
| The main objective of immunotoxicology is to protect humans and animals against the harmful effects of chemical factors present in the environment, to develop and check immunotherapeutic products. Harmful effects of environmental factors can arise from direct or indirect action of xenobiotics on the immune system (Immune cells and Antibodies) or with products obtained through biotransformation. Modification of self-antigens by chemicals or their metabolites.. To meet this objective the veterinary immunotoxicology must look for solutions to at least 3 principal tasks: (1) Development of standard and reliable methods or adaptation of existing immunotoxic tests considering those physiological-anatomical specifities of individual farm animal species which can result in different immunotoxicity. (2) Determination of the degree of immunotoxic potential of any pesticide to animals before it is introduced into practice. (3) Monitoring of the immune system of animals exposed to various pesticides, drugs and other xenobiotics. |
| We have no organization in SR dealing with the influence of xenobiotics on the immune system. Only individual enthusiasts have been making an effort to solve some partial tasks without sufficient support from state bodies. We must emphasize immediate necessity of establishing a separate branch of science – veterinary toxicology and pharmacology - that could create the necessary space for immunotoxicology of pesticides and drugs in farm and free living animals. The minimization of negative economical impact of pests in agriculture and forestry is related to the necessary broad-spectrum application of protective preparations and pharmacological substances. |
II. IMMUNOTOXICOLOGY AS A BRANCH OF SCIENCE |
| Immunotoxicology is relatively new interdisciplinary scientific field focused on identification and analysis of chemical and, in a broader sense, also physical and biological factors of the environment which can cause unwanted and usually incidental immunomodulations [18]. Similar subjects are studied also by immunopharmacology. As opposed to immunotoxicology, immunopharmacology investigates the immunomodulative effects of various substances that are applied intentionally for therapeutic purposes. The objective of immunotoxicology is to protect humans and animals against the harmful effects of chemical factors present in the environment, to develop and check immunotherapeutic products and introduce and evaluate the methods intended for determination of interactions between the factors of the external environment and the immune system [18]. Harmful effects of environmental factors can arise from:- |
| i. Direct or indirect action of xenobiotics on the immune system or of products obtained by their biotransformation. |
| ii. Induction of the immune response to xenobiotics or their metabolites. |
| iii. Modification of self-antigens by chemicals or their metabolites [5]. |
| iv. In addition, immunotoxicology also investigates the properties of new immunotherapeutic pharmacological products prepared via recombinant DNA techniques (interleukins, interferons, growth factors, anti-inflammation drugs, neuroendocrine hormones, neuropeptides) with regard to their immunotoxic potential and safety of their use [42]. Another specific domain of immunotoxicology deals with immunotoxins, the substances isolated from bacteria and plants the general property of which is the inhibition of proteosynthesis (diphtheria toxin, Pseudomonas exotoxin, ricin, ribosome-inactivating protein and others), as well as with conjugates of monoclonal antibodies and their use mostly in antitumour immunotoxin therapy [49]. Another not unimportant field of immunotoxicology is the development of methods which can be used to investigate the interactions between the external environment and the immune system. |
III. NATURE OF SUBSTANCES EXHIBITING IMMUNOTOXIC EFFECTS |
| Immunotoxicants are factors of the external environment which cause significant changes (modulation) in the immune mechanisms in humans and animals [18]. Zbinden [52] used the term immunomodulators to characterize a group of substances of different origin which affect the immune system and includes environmental chemical contaminants like pesticides, industrial emissions, drugs including potentially sensitising impurities in products manufactured by recombinant DNA and other biotechnological technologies [13] and physical factors (UV-B light, electromagnetic field - Luster et al. 1990). The action of immunotoxicants results in the reduced reactivity of the immune system - immunosuppression(e.g. PCB, lead, cadmium) [41], [44] or, on the contrary, in its excessive reactivit hypersensitivity (polycyclic aromatic compounds, organophosphate insecticides and others – [32]). Some substances (e.g. nickel and mercury) can participate in the development of autoimmune diseases [33]. The present interest focuses on possible immunologic effects of indoor pollutants. This involves not only chemicals but also bioaerosols, such as viruses, bacteria, moulds, algae and protozoa, as potential sensitizing agents or mediators of infectious diseases [33]. |
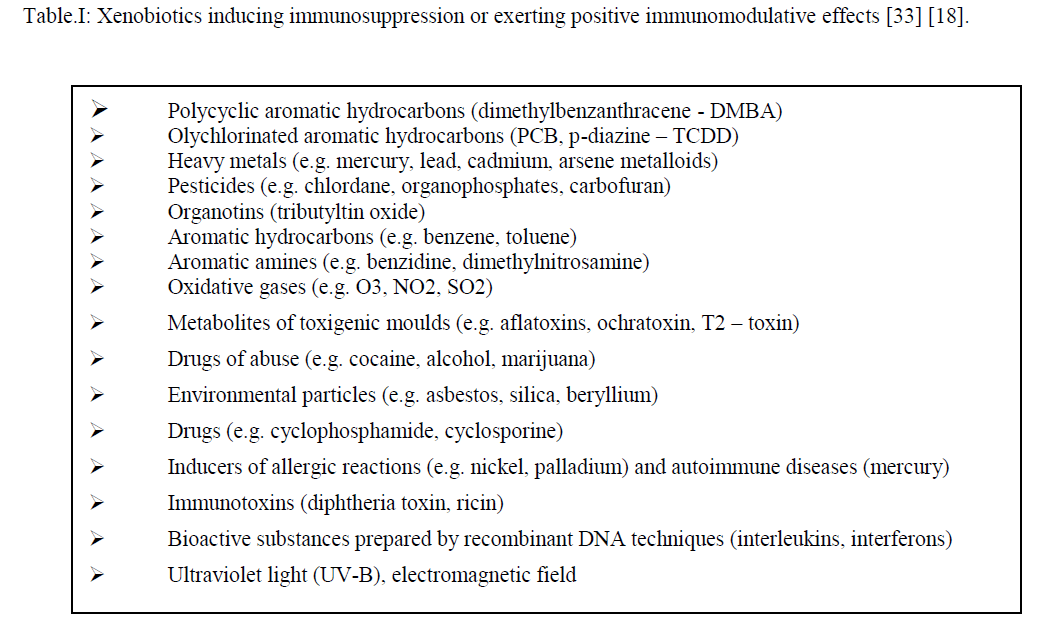 |
IV. SPECTRUM OF THE EFFECT OF IMMUNOTOXICANTS |
| The susceptibility of the immune system to immunotoxicants depends on both the properties of the respective chemical and complex nature of the immune system (antigen recognition and processing, cellular interactionscooperation, regulation and amplification, activation and differentiation of cells and production of mediators by various cell types). The interactions of xenobiotics with the immune system can be affected also by additional factors, such as malnutrition, stress and genetic predisposition [32]. From the point of view of respective mechanisms of immunotoxicants we recognize systemic (interaction with one or more components of the immune system) and local effects (e.g. decreased local lung immunity) and also some selective effects on target cells (B-lymphocytes, Tlymphocytes, macrophages). |
IV.I. SYSTEMIC IMMUNOSUPPRESSIVE ACTION |
| Systemic immunosuppression affects the activity of the immune system as a whole and develops frequently as a result of action of various chemical agents. The most detailed studies of this form of immunotoxicity were carried out in rodents. The systemic immunosuppression is indicated by the following: (a) altered weight and histology of lymphoid organs ( b) quantitative changes in cellularity of the lymphoid tissue, peripheral blood leukocytes and bone marrow,( c) impairment of cell function at the effector or regulatory level, (d) increased susceptibility to infectious agents or transplantable tumours [33]. Some examples of the immunosuppressive mechanisms are:- |
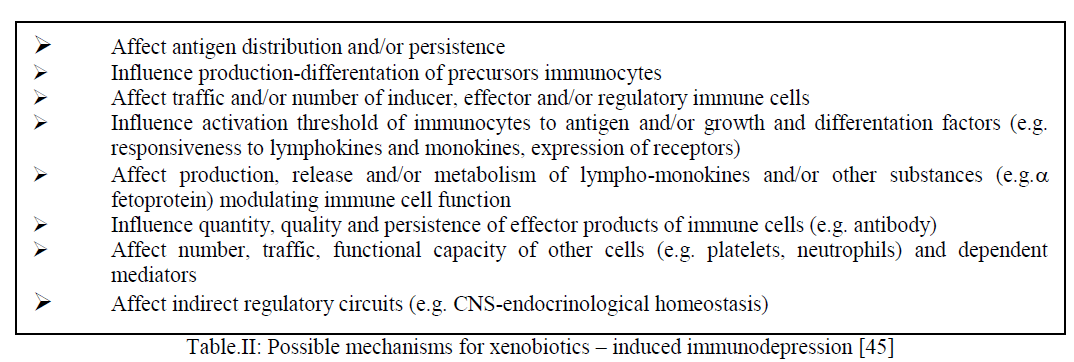 |
IV.II. LOCAL IMMUNOSUPPRESSIVE ACTION |
| Local immunosuppression induced by xenobiotics is most frequently demonstrated in lungs and on skin in both humans and animals. |
| i. Lungs |
| Pathogenesis of a number of pulmonary diseases, such as fibrosis, granulomatosis and bronchial asthma, is associated with inhalation of pollutants. The pulmonary diseases which are conditional upon local damage to the immune system can be induced by various agents, such as oxidizing gases (ozone, NO2, SO2) or fine solid particles (silica, beryllium, asbestos, coal dust, sheep wool and others). Numerous studies point to the fact that the progress of pulmonary diseases is related to the post-activation release of cytokines, predominantly interleukin 1 (IL-1), tumour necrotizing factor (TNF-ïÃÂá, TNF-ïÃÂâ) platelet-derived growth factor (PDGF), growth transforming factor beta (TGF-ïÃÂâ), by pulmonary macrophages [33]. In addition to cytokines, alveolar macrophages also produce various short-term acting products which can contribute to a decreased resistance of lungs to infection and inflammation. This refers particularly to the reactive forms of oxygen, such as superoxide and hydrogen peroxide as well as to metabolites of arachidonic acid. Lungs also contain considerable numbers of NK cells (natural killers), probably as a result of evolutionary development, for the purpose of protection of lungs against pulmonary tumours the development of which may be induced by inhaled carcinogens. Several agents, such as fosgen, decrease the activity of pulmonary NK-cells. |
| ii. Skin |
| Similar to the lungs, the skin is another potential place of entry of many xenobiotics. Skin reactions can acquire the form of specific immune response (contact hypersensitivity) or nonspecific inflammation response (contact irritation), both conditional upon several similar pro-inflammation cytokines. Immunologically active cells and their soluble mediators penetrate from the circulatory system into the skin as a response to the stimulus induced by xenobiotics. Besides that, other dermal cells (such as Langerhans cells) which participate in the immune reactions can be activated in response to dermal stimuli. Under the action of exogenous factors, such as ultraviolet radiation (UV) or some chemicals (dimethyl benzanthracene), they can disappear or lose their function [33]. Keratinocytes as cells prevalent in epidermis also play important role in the immune and inflammatory response. Keratinocytes induced by UV-B radiation, chemical irritants, sensitizing compounds and some pharmacological substances produce a wide scale of cytokines: IL-1, granulocyte and macrophage colonies stimulating factor (GM-CSF), IL-6, IL-8, TGF-ïÃÂá, TGF-ïÃÂâ, TNF-ïÃÂá and IL-3. The basic response of keratocytes consists in the production and secretion of IL-1ïÃÂá and TNF-ïÃÂá which results in the expression of leukocyte adhesive molecules on the surface of dermal endothelial cells (e.g.VCAM-1). IL- 8 is an important chemotactic and activating substance affecting polymorphonuclear neutrophils (PMNL). In case of antigenic character of the stimulant (e.g. nickel sulphate), an increased number of mononuclear cells and subsequent participation of T-helper cells is characteristic of the response. This leads to significant release of IFN-ïÃÂç and TNF-ïÃÂá with a resultant enhancement of the response [33]. |
IV.III. EXCLUSIVE EFFECT ON TARGET CELLS |
| Some xenobiotics exhibit more intensive selective effect on certain type of cells or some cells exhibit primary susceptibility to the action of such substances. Table shows the examples of the effect of immunosuppressive substances on the function of macrophages and monocytes [45]. |
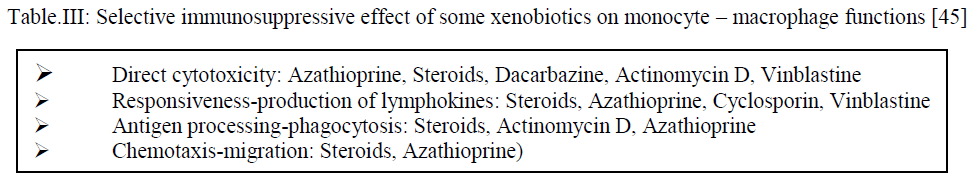 |
IV.IV. HYPERSENSITIVITY MEDIATED BY XENOBIOTICS |
| Chemically induced hypersensitivity, as a manifestative stage of immunotoxicity, aroused great interest of clinicians [5]. Contact hypersensitivity reaction is the principal demonstration of hypersensitivity induced by xenobiotics [2]. Such a reaction develops after the contact of various substances with body surface during which xenobiotics bind to skin proteins and change from incomplete antigens (haptens) to complete ones capable of inducing the immune response. The sensitization that a result from primary exposure to xenobiotics develops for several days, however, once it is established, it lasts for many years or for life. When the activated T-lymphocytes are repeatedly exposed to allergens, they release numerous cytokines (macrophage activating factor - MAF, macrophage chemotactic factor - MCF and macrophage inhibiting factor - MIF) which attract additional macrophages to the inflammation foci and activate them. The activated macrophages release proteolytic enzymes which can damage the tissues. Eczema are produced clinically in the place of contact with an allergen. The acute phase is manifested as erythema with swelling and formation of vesicles while production of papulae and scales and focal pruritus predominates in the acute phase [11]. Contact hypersensitivity depends on additional physiological and pharmacological factors, such as permeability of skin which affects directly the antigen dose uptake [2]. There are records of contact dermatitis caused by various components of paint coats (polycyclic aromatic compounds), pesticides, different chemicals (e.g. formaldehyde, epoxide, Peruvian balm, lanolin, stains and others), drugs (neomycin) and plants, such as poison [11]. Particular attention was paid to contact dermatitis caused by nickel which is a frequent constituent of various articles (costume jewellery, jewellery, metal eyeglass frames, dog collars, etc.). Nickel dissolves in weakly acidic environment of sweat and its salts have high affinity to various proteins, including albumin and proteins of the complement system which creates conditions for the development of hypersensitivity reaction [19] [2]. The development and course of contact hypersensitivity is also affected by genetic factors (particularly genes encoding MHC class II antigens) and additional factors that can affect the presentation of antigens [2]. Speaking about immune mechanisms of contact hypersensitivity, one must distinguish them from pseudoallergies which resemble the allergic reaction. The group of diseases which, according to their etiopathogenesis, includes also type IV cellular hypersensitivity comprises also hypersensitive pneumonitis induced by inhalation of various allergens. The hypersensitive pneumonitis has been described under different names, such as farmer’s lungs, furrier’s lungs, pigeon breeder’s disease, cheese washer’s disease. The initial phase ofhypersensitive pneumonitis is characterised by formation of immunocomplexes in the lung interstitium composed of IgA or IgG antibodies and the inhaled pathogen. In case of repeated exposure, T-lymphocytes are also involved in the immunopathogenic mechanisms and granulomatous interstitial pneumonia develops [11]. |
IV.V. AUTOIMMUNE DISEASES |
| One of the characteristic features of the immune system is the recognition and the development of immune response to various xenogenous antigens present in the external environment and the absence of immune response to the own tissues. Some degree of autoreactivity is a normal feature of the immune system physiology because somatic proteins, many carbohydrates, lipids and nucleic acids are potential antigens capable of inducing humoral and cellular immune responses [27]. The mechanism that prevents the induction of an immune response to own body components and prevents potential autoreactive lymphocyte clones from entering into reactions is called tolerance. An impairment of the immunotolerance caused by various factors results in autoimmune diseases [27]. One of the possible ways of the development of autoimmune disease is the reaction of autoantibodies with xenogenous substances which are bound to own cells or structures of the organism, for example, autoimmune haemolytic anemia can develop as a result of interaction of antibodies with erythrocyte-bound penicillin [22] or pesticide dieldrin. Autoimmune glomerulonephritis, which develops as a result of deposition of circulating immunocomplexes (mercury and gold) in the basal glomerular membrane can serve as another example. The autoimmune process was demonstrated as glomerulonephritis, lymphadenopathy, production of antinuclear antibodies and significant increase in the level of IgE. Some other autoimmune diseases, induced by HLA-molecule-associated xenobiotics, were also described: hydralazine induced systemic lupus erythematosus [4], D-penicilamine and aurothiomalate-induced nephropathy [51], selective IgA deficiency induced by diphenyl hydantoin [43], production of autoantibodies of various specifity induced by Spanish toxic oil [49] and serious scleroderma-resembling lesions induced by vinyl chloride [7]. |
V. WAYS OF DETERMINATION OF IMMUNOTOXIC PROPERTIES OF XENOBIOTICS |
| The immunotoxic mechanism of xenobiotics is implemented either through direct action of the chemical with the immune cells and their membrane receptors, affecting the production of cell mediators and antibodies, or indirectly, through secondary effects on important central organs such as liver (hepatotoxicity - production of hepatoproteins with immunomodulative properties) and the neuroendocrine system (neuroendocrine toxicity - chemically induced changes in hormones or neuroactive substances with immunomodulative properties). The direct and indirect action can produce a combination of both effects [18]. Several methods were developed to determine the immunosuppressive or immunostimulative action of xenobiotics using the following: dose and time-dependence of response, toxicokinetic, determination of direct and indirect effects on the immune system as well as biological relevance of in vitro tests to the effects of agents in vitro. The examinations should provide answers to the following 3 questions: 1. Whether the xenobiotic can alter the immune functions, 2. If yes, what concentration and exposure time is required to induce a response, 3. Whether the repeated exposure to the xenobiotic mentioned causes activation of the immune mechanisms resulting, for example, in an autoimmune reaction [5]. In searching for the answers to the questions presented the following factors are of essential importance: (a) selection of species of experimental animals, (b) determination of exposure interval, (c) dose, and (d) setting up the panel of the tests which can be used to evaluate the immune status of experimental animals [52]. |
V.I. MODEL EXPERIMENTAL ANIMALS, CELLS AND CELL CULTURES |
| The tests of immune function alterations have been carried out mostly on rodents because of their defined genetic characteristic, suitability for host resistance tests and resemblance between their immune system and the immune system of humans. Guinea pigs are frequently used as experimental models in testing the contact delayed hypersensitivity reaction [5]. The selection of animals should correspond to the objective of the experiment, i.e. dogs should be selected for evaluation of drug effects, rabbits for teratogenesis and so on [14]. In view of the fact that the majority of experiments have been focused on the man there is some controversy regarding the application of results obtained in rodents to humans for the purpose of application of immunotoxicological data obtained in mice to other animal species or to the man one can use the method of xenogenous extrapolation. It is necessary to consider the differences in the susceptibility of individual mice strains to immunotoxicants ranging from high susceptibility to relative resistance [18]. Immunotoxicological tests were carried out also on rabbits [1], [21], golden hamsters [8], pigs of small breeds [18], dogs [28], sheep [39] and monkeys [18]. In addition to mammals, chickens [6] and some fish species [46], [29] are also frequently used as models. The studies of direct immunotoxic effect employ also cell cultures [18] and deal with in vitro effects of xenobiotics on leukocytes isolated from the peripheral blood [24]. An effort has been made recently to prepare new animal models (knockout mice, transgenic animals and similar). Mice with severe combined immunodeficiency syndrome (SCID mice) were transplanted with human immune stem cells. Such a xenogenous mosaics provided mice containing intact human immune system cells. However, the question of neuroendocrine-immune and hepaticimmune interactions within the mice mentioned in comparison with humans remains unclear [18]. |
V.II. EXPOSURE INTERVAL |
| The time needed to induce immune dysfunction, depends on the type of immunological damage, chemical characteristics of the substance tested, toxicokinetic of the compounds and functional reserve of the loaded immune parameters. In general, in case of subcutaneous repeated-exposure regimen used in animals at the beginning of sexual maturity, approximately 14-30 days are needed before the chemically induced effect on immunocompetence is recorded [14]. Up to this date, only few chronic exposure experiments were carried out. The long-term acting low levels of xenobiotics used in them can have no effect on animals; however, on the other hand, more serious or persistent effects of chronic action could be demonstrated in comparison with the subacute action. |
V.III. SELECTION OF THE DOSE |
| Is a principal step in immunotoxicological experiments one should avoid to high doses causing apparent toxicity. The selection of the dose should be based on data about the effect of chemicals on general toxicological parameters (LD50, LD10, type of acute or subchronic toxicity in dependence on the dose). As a rule, three exposure levels are recommended for determination of the dose-dependent effect of xenobiotics. An ideal situation occurs when the lowest dose used is lower than LD10 and does not result in mortality. Under normal conditions, the lowest dose should not influence the immune functions [14]. |
V.IV. METHODS |
| The methods commonly used to evaluate immunotoxical effect of xenobiotics recognize non-functional and functional tests [48]. Nonfunctional tests provide information mainly about the changes in the lymphoid tissue, number of peripheral lymphocytes and monocytes, level of total globulins, cytokines, etc. [14], [48], [33]. Functional tests (Table) reflect in greater detail the situation in vivo because they focus on the direct assessment of phagocytic and antigen-specific components of immunity. The evaluation of immunotoxicological action of xenobiotics requires a spectrum of methods that can provide as detailed as possible information about the immune system status. In this process one must consider that some immune status parameters are not necessarily affected by xenobiotics while others are in turn affected significantly. This is suggested by a range of results obtained in the course of subchronic intoxication of sheep with some pesticides and during acute intoxication of sheep with heavy metals [36] [37] [39]. |
VI. ANIMAL HEALTH PROBLEMS WITH RESPECT TO PESTICIDE TOXICOLOGY |
| The reality of today is that pesticides and heavy metals from emissions are dominant components of the chemical load on the environment of man and animals [23]. One can assume that further improvement in technology of trapping the industrial emissions will minimize gradually the problem of ecotoxicology of heavy metals. Gradual decline in residues of chlororganic pesticides mentioned is related to the fact that insecticides-acaricides based on DDT and technical HCH were banned in 1975 and those based on HCB in 1980 [9] [23]. Sporadic cases of acute intoxications of animals with pesticides could be prevented by strict observation of the valid legislative standards determining the application and storage of protective chemicals in the agriculture-forestry practice and in municipal hygiene. However, it is obvious that chronical harmful effects of pesticides on the animal kingdom must also be considered with regard to distant future. To be specific, the protective chemicals are the principal means of protection of agricultural crops against pests and disease agents and controlling the weeds. |
VI.I. VETERINARY IMMUNOTOXICOLOGY OF PESTICIDES |
| Immunotoxicology in veterinary medicine deals with the problems arising from dominant ecological toxicants, such as pesticides. From the medical point of view we can refer to it as ecoimmunotoxicology. According to reliable literary sources, almost none of the active ingredients present in pesticides that are used in our country were subject to immunotoxicological testing. The exposure of animals to residual concentrations of pesticides can lead to immunosuppression either directly or with participation of stress mechanisms (hunger, thirst, unfavourable microclimate conditions, long distance transport fatigue and others) and of the neuroendocrine system. Immunosuppression results in defective immunological supervision (e.g. defective maturation of cells) conducive to the development of tumours. However, with regard to the length of life of animals, more typical of them are changes in the length of life, increased susceptibility to infectious diseases and decreased immune response to vaccination. Potential consequences of immunotoxicity of pesticides can be divided to three groups: 1. Direct immunotoxicity (connected mainly with immunodepression), 2. Hypersensitivity reactions, 3. Autoimmune reactions [16]. |
VI.II. PESTICIDE-INDUCED IMMUNOSUPPRESSIVITY |
| Immunoinsufficiency was studied with regard to chlororganic insecticides (DDT, HCH, heptachlorine – [26] [10], [35], organophosphates [38] [47] pyrethroid insecticides (allethrin, cypermetrin, fenpropathrin, permethrin – [15] herbicides (atrazin 2,4- D, 2,4,5 - T – [15] [20] fungicides (pentachlorphenol - PCP - Lang Mueller –[12]) and carbamates (aldicarb – [3]). The possibility of long-lasting consequences of low doses of pesticides on the immune system in the course of chronic exposure to pesticides cannot be excluded [40]. The selection of immunotoxicity biomarkers is one of the priorities of correct interpretation of minor changes in the immune system that are not easily reflected in measurable health indices [17]. If it is possible to provide biological proof of the immunosuppressive effect in the experimental model then the proof of clinical consequences requires simultaneous exposure e.g. to microbial or viral agents, or inoculation with neoplastic cells. Immunotoxic effects of pesticide preparations could result sometimes from by-products of the synthesis of active ingredients used in pesticides, or products of their biotransformation. The immunotoxic potential of pesticides is also related to the requirement on rational planning of epidemiological studies [34], [40]. Some partial results of epidemiological studies related to the extent impairment of to the immune system of humans exposed to chronic action of pesticides could provide preliminary information which can help in evaluation of the effect of residual concentrations of effective ingredients in pesticides, mainly on immunosuppression in animals which is most frequently associated with increased susceptibility to infectious diseases and decreased response to vaccination. |
VII. URGENT TASKS OF VETERINARY IMMUNOTOXICOLOGY |
| The testing of emergency immune system of animals in the production of ecological food of animal origin and increasing production-reproduction capabilities of animals takes priority over all other objectives of veterinary medicine. Because of that, out of all obligatory OECD tests determining the toxicological risk of pesticides to animals [25] the greatest emphasis should be put on their possible immunotoxicity. To meet this objective the veterinary immunotoxicology must look for solutions to at least 3 principal tasks: (1) Development of standard and reliable methods (correct laboratory practice in veterinary immunotoxicology) or adaptation of existing immunotoxic tests considering those physiological-anatomical specifities of individual farm animal species which can result in different immunotoxicity. (2) Determination of the degree of immunotoxic potential of any pesticide to animals before it is introduced into practice. (3) Monitoring of the immune system of animals exposed to various pesticides, drugs and other xenobiotics. |
VIII.CONCLUSION |
| One must agree with the statement of immunologists that the situation in our country is completely different. We have no organization in SR dealing with the influence of xenobiotics on the immune system. Only individual enthusiasts have been making an effort to solve some partial tasks without sufficient support from state bodies. In the education sphere, we are aware of the absence of an appropriate system for underegraduate and graduate education of immunotoxicologists. It is well a known fact that education of such specialists is a long-term process. Here we must emphasize immediate necessity of establishing a separate branch of science – veterinary toxicology and pharmacology - that could create the necessary space for immunotoxicology of pesticides and drugs in farm and free living animals. The minimization of negative economical impact of pests in agriculture and forestry is related to the necessary broadspectrum application of protective preparations and pharmacological substances. In this sense the immunotoxicological testing resembles “extinguishing of fire”. One must consider the requirement “not to allow the fire to flare up”. However, this is the task other specialists are faced with: correct agrotechnical practice (early and thorough ploughing, sowing and similar) efforts of genetics, breeders, entomologists - cultivation of crop varieties resistant to insect, pathogenic moulds and so on. |
References |
|