e-ISSN: 2319-9849
e-ISSN: 2319-9849
Onkar Chand*, Lalita Chopra, Shashi Kant Tiwari
Department of Chemistry, Chandigarh University Gharuan, Punjab, India
*Corresponding Author:
Received: 30-Oct-2023, Manuscript No. JCHEM-23-118844; Editor assigned: 02-Nov-2023, Pre QC No. JCHEM-23-118844 (PQ); Reviewed: 16-Nov-2023, QC No. JCHEM-23-118844; Revised: 06- January-2025, Manuscript No. JCHEM-23-118844 (R); Published: 13-January-2025, DOI: 10.4172/2319-9849.14.1.001
Citation: Chand O, et al. Review on Synthetic Approaches and Reported Polymorphs for Pazopanib Hydrochloride (Votrient), an Anticancer Drug. RRJ Soc Sci. 2025;14:001.
Copyright: © 2025 Chand O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Chemistry
Cancer advert to rapid and uncontrolled growth of cells which expand to different parts of the body through blood or lymph and the drugs which inhibits the abnormal growth of cells or kill the cancer cells are known as anti-cancer drugs. In the recent years, there is a remarkable growth in the development of anticancer drugs. This article reviews the literature on synthetic routes and different polymorphic forms of one of the anti-cancer drug named “VOTRIENT (Pazopanib)” a Tyrosine Kinase Inhibitor (TKI). A systematic review of different articles, patents, and official assessment reports of different agencies shows that Novartis is the inventor of Pazopanib drug substance, and its route of synthesis consists of mainly four steps starting from 3-methyl-6-nitro-1H-indazole. The marketed polymorphic form of Pazopanib hydrochloride prepared by inventor is anhydrous crystalline polymorphic form 1. This review gives an insight of different existing route of synthesis and polymorphic forms of Pazopanib and its different reported salts.
Anticancer drugs; Pazopanib; Synthesis; Polymorph; Stability
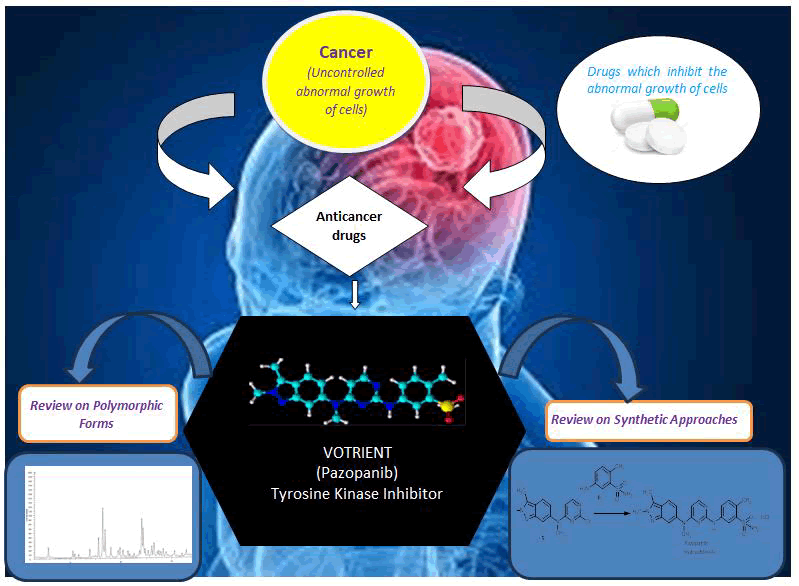
GRAPHICAL ABSTRACT
“Cancer is a rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs. This process is referred to as metastases.”
Cancer medically known as malignant neoplasm [1], develops when cells start growing in an unregulated way. Malignant neoplasm can spread into nearby tissues and to other parts of the body through blood or lymph. More than 200 different types of cancer are reported till now. Cancer can be divided into types based upon where it begins. Four main types of cancer [2] are mentioned below in Figure 1.
Figure 1. Type of cancer.
Carcinomas is a type of cancer which begins in the skin or in the tissue, Sarcomas begins in the tissues that support and connect the body, Leukaemia is a cancer of the blood cells and Lymphomas begins in the lymphatic system.
Considerable discoveries have been made in the field of cancer treatment in past few decades. Data reveal that an average 5-10 new anticancer drugs (also known as antineoplastic drugs) are being approved every year. Historically, anticancer drugs are mainly categorized [3] as tabulated in Table 1.
| Anticancer drugs | Examples |
| Alkylating agents | Altretamine, Bendamustine, Carmustine, Dacarbazine, Lomustine, Mechlorethamine, Procarbazine, Temozolomide, Thiotepa, Trabectedin |
| Antibiotics, cytotoxic agents | Bleomycin, Dactinomycin, Doxorubicin, Epirubicin, Idarubicin, Mitoxantrone, Plicamycin, Valrubicin |
| Antimetabolites agents | Azathioprine, Azacitidine, Capecitabine, Cladribine, Decitabine, Floxuridine, Fludarabine, Gemcitabine, Mercaptopurine, Trimetrexate |
| Biologic response modifiers | Aldesleukin, Denileukin Diftitox, Interferon Gamma |
| Histone deacetylase inhibitor | Belinostat, Panobinostat, Vorinostat |
| Hormonal agents | Abiraterone, Anastrozole, Apalutamide, Cyproterone, Exemestane, Flutamide, Fulvestrrant, Lanreotide, Letrozole, ,Raloxifene, Triptorelin |
| Monoclonal antibodies | Alemtuzumab, Bevacizumab, Cemiplimab, Daratumumab, Elotuzumab, Inotuzumab, Nivolumab, Ozogamicin, Pertuzumab, Rituximab |
| Protein kinase inhibitors | Acalabrutinib, Abemaciclib Binimetinib, Cabozantinib, Dacomitinib, Fedratinib, Gefitinib, Ibrutinib, Lapatinib, Nilotinib, Palbociclib, Pazopanib, Pemigatinib, Ribociclib, Sunitinib, Tucatinib, |
| Taxanes and topo isomerase | Cabazitaxel, Docetaxel, Paclitaxel Etoposide, Irinotecan, Topotecan |
| Vinca alkaloids | Vinblastine, Vincristine, Vinorelbine |
| Miscellaneous | Bexarotene, Everolimus, Hydroxyurea, Mitotane, Omacetaxine, Pegaspargase, Telotristat, Thalidomide |
Table 1. Historical categorization of anticancer drugs.
Protein kinase inhibitors are the substances that inhibit the action of protein kinase enzymes which regulate cell growth. In recent years there is a significant growth in the elaboration of protein kinase inhibitors such as VEGF/VEGFR inhibitors, Bruton’s Tyrosine Kinase (BTK) inhibitors and Cyclin Dependent Kinase (CDK4/6) inhibitors. (CDK4/6) inhibitors (Palbociclib, Abemaciclib and Ribociclib) are approved for treatment of patients (HR+/HER2-) advanced breast cancer by targeting the cell cycle. Recently, in India Cipla introduce Palbociclib capsules (125 mg, 100 mg and 75 mg) which inhibits the phosphorylation of RB protein, by blocking cell cycle progression. Some representative Protein Kinase Inhibitors are listed in Table 2.
| Protein kinase inhibitors | Structure | Drug class |
|---|---|---|
| Abemaciclib (Brand name: Verzenio®) | 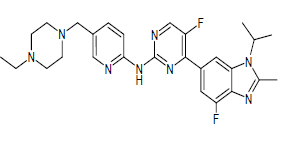 |
Cyclin-Dependent Kinases (CDK4/6)) inhibitor |
| Palbociclib (Brand name: Ibrance®) | 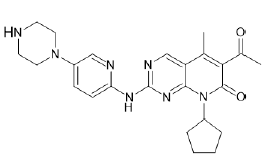 |
Cyclin-Dependent Kinases (CDK4/6) inhibitor |
| Acalabrutinib (Brand name: Calquence®) | 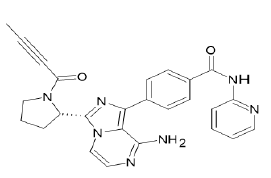 |
Bruton’s Tyrosine Kinase (BTK) inhibitor |
| Ibrutinib (Brand name: Imbruvica®) | 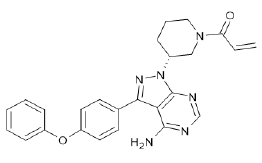 |
Bruton’s Tyrosine Kinase (BTK) inhibitor |
| Imatinib (Brand name: Gleevec®) | 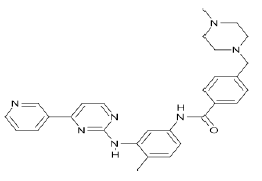 |
BCR-ABL tyrosine kinase inhibitor |
| Nilotinib (Brand name: Tasigna®) | 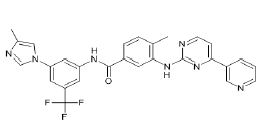 |
BCR-ABL tyrosine kinase inhibitor |
| Gefitinib (Brand name: Iressa®) | 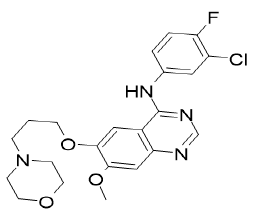 |
EGFR tyrosine kinase inhibitor |
| Erlotinib (Brand name: Tarceva®) | 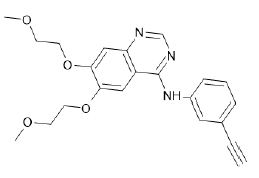 |
EGFR tyrosine kinase inhibitor |
| Dacomitinib (Brand name: Vizimpro®) | 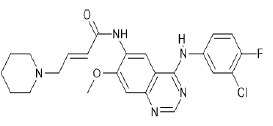 |
EGFR and HER2 inhibitor |
| Lapatinib (Brand name: Tykerb®) | 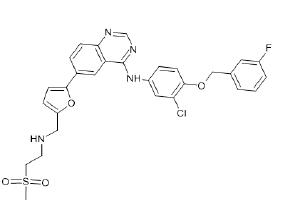 |
EGFR and HER2 inhibitor |
| Pazopanib (Brand name: Votrient®) | 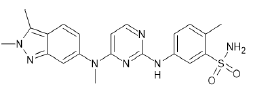 |
VEGF/ VEGFR inhibitor |
| Sunitinib (Brand name: Sutent®) | 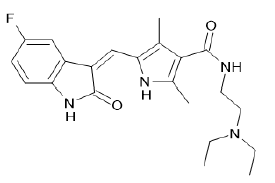 |
VEGF/ VEGFR and Multi-kinase inhibitor |
| Cabozantinib (Brand name: Cometriq®) | 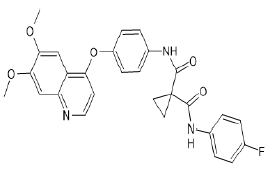 |
VEGF/VEGFR and Multi-kinase inhibitor |
| Binimetinib (Brand name: Mektovi®) | 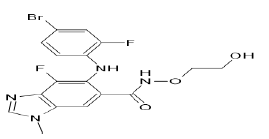 |
Multi-kinase inhibitor |
| Fedratinib (Brand name: Inrebic®) | 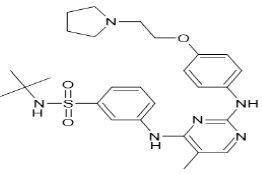 |
Multi-kinase inhibitor |
| Rucaparib (Brand name: Rubraca®) | 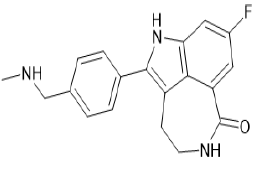 |
Poly (ADP-ribose) poly-merase inhibitor |
Table 2. Representative protein kinase inhibitors.
There are several other Protein Kinase Inhibitors which are recently approved by FDA or under clinical trials such as Abrocitinib, Almonertinib, Amivantamab, Asciminib, Avapritinib, Capmatinib Tabrecta, Defactinib, Delgocitinib, Deucravacitinib, Filgotiniv, futibatinib, Infigratinib, Lazertinib, Margetuximab, Mobocertinib, Orelabrutinib, Pacritinib, Paxalisib, Pemigatinib, Pyrotinib, Ripretinib, Savolitinib, Selumetinib, Surufatinib, Tepotinib, Tirabrutinib, Tucatinib.
A new anticancer drug undergoes an extensive development and approval process, involving many significant stages and milestones. The quest for new drug starts with accession of new novel compounds through various computational methods for screening the potential compounds for further development studies. After screening potential compounds, their toxicity and anticancer activities are examined, followed by pre-clinical and clinical trials to ensure their safety and efficacy to get it approved for human use. During development of new drugs, the main motive of innovator is rapid preparation of drug substance with optimum efficacy and quality to capture market as early as possible. For development of generic drugs, the strategies are different. Wherein a Quality by Design (QbD) approach is applied to develop a cost effective, scalable, environment friendly, non-infringing patent free process with established impurity profiling and polymorphic forms [4]. In a document “The FDA critical path initiative and its influence on new drug development” specific parameters in the development of generic drugs are defined [5].
VOTRIENT® (Pazopanib) also called GW786034 [6] is a Tyrosine Kinase Inhibitor (TKI) [7]. Pazopanib is developed and marketed by Novartis/GlaxoSmithKline and was approved by FDA on October 19, 2009 [8-10]. VOTRIENT® (Pazopanib) targets tyrosine kinase activity associated with vascular endothelial growth factor receptor (VEGFR)-1, -2 and -3, stem cell factor receptor (c-KIT), cytokine receptor, leukocyte-specific protein tyrosine kinase, transmembrane glycoprotein receptor tyrosine kinase and platelet-derived growth factor receptor (PDGFR)-α, and (PDGFR)–β. Lot of these receptor targets are part of angiogenesis pathway and are essential for growth and survival of tumour. Pazopanib targets proteins (called tyrosine kinases) on the peripheral of cancer cells, as well as targets within the cell and bring out significant alterations in these receptor targets which helps in tumour regression [11]. Pazopanib is administered as the hydrochloride salt. In patent application WO 2005/105094 A2 cancer treatment method is described including administration of pyrimidine derivatives [12]. Patent application WO 2007/064753 has disclosed Pazopanib for various type of cancer treatment [13].
The development work for improvement in existing routes and for developing new synthetic route for VOTRIENT® (Pazopanib) is still under progress in different institutes and pharma industries. This article is related to literature review focusing on anticancer drug namely Pazopanib with inventor route of synthesis, different innovative synthetic routes other than inventor’s route and related to different polymorphic forms reported in the literature (Table 3).
Product profile
| Chemical name | 5-[[4-[(2,3-dimethylindazol-6-yl)-methylamino] pyrimidin-2-yl]amino]-2-methylbenzene sulfonamide |
| Chemical structure | 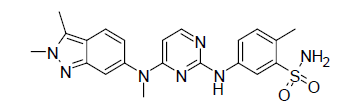 |
| Molecular formula | C21H23N7O2S |
| Molecular weight | 437.5 as Pazopanib base and 473.99 as Pazopanib hydrochloride |
| CAS no. | 444731-52-6 for Pazopanib base and 635702-64-6 for Pazopanib hydrochloride |
| Drug central ligand | 4118 |
| Drug bank accession number | DB06589 |
| INN, USAN, JAN | Pazopanib, pazopanib hydrochloride |
| Proprietary name | Votrient |
| Innovator/marketed by | Novartis/glaxosmithkline |
| Therapeutic category | Antineoplastic/anticancer (Renal cell carcinoma) |
| Doses forms | Film coated tablets |
| Strength | 200 and 400 mg |
| Maximum daily dose | 800 mg (equivalent to Pazopanib free base), (Pazopanib is administered as hydrochloride salt) |
| Polymorphism (innovator) | Anhydrous, crystalline Form-1 (Pazopanib hydrochloride) |
| Pharmacokinetics | Absorption: 2-8 hours post dose, Bioavailability: 21% (1-39%), Protein binding: >99.5%, Elimination half-life: 30.9 ± 4 hours, Metabolism: Metabolized by CYP3A4 512 with a minor contribution from CYP1A2 and CYP2C8, Excretion: Faeces (primary), Urine (<4%) |
| Genotoxicity | Non-mutagenic and non-clastogenic |
Table 3. Product profile.
Overview of synthetic routes
VOTRIENT® (Pazopanib) is a pyrimidine derivative consisting of three main structural units as shown in Figure 2.
Figure 2. Structure of pazopanib.
To synthesize pazopanib different approaches are applied to couple these three main structural units. In literature, there are number of different synthetic routes for synthesis of pazopanib are reported [14-16].
Innovator’s route of synthesis
In patent application publication nos. US 2004/0242578 A1 [17], and US 2012/0277258 A1 [18], patent nos. US 7,105,530 B2 [19] and US 8,114,885 B2 [20], Assignee: SmithKline Beecham Corporation, manufacturing route for pyrimidine derivatives useful as VEGFR2 inhibitors was described wherein synthesis of pazopanib hydrochloride involves the following steps (Figure 3):
Figure 3. Synthesis of pazopanib hydrochloride using acetone, trimethyloxonium tetrafluoroborate.
In this route of synthesis, 3-methyl-6-nitro-1H-indazole (compound 1) is first methylated with trimethyloxonium tetrafluoroborate followed by reduction of nitro group to form 2,3-dimethyl-2H-indazol-6-amine (compound 2), then 2,4-dichloropyrimidine (compound 3) is reacted with compound 2 in the presence of sodium bicarbonate to generate N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine (4). Compound 4 is methylated with methyl iodide in the presence of cesium carbonate to get N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amine (compound 5) and this further reacted with 5-amino-2-methylbenzenesulfonamide (compound 6) to afford Pazopanib hydrochloride. This route looks safe and commercially viable. For reduction of nitro group Pd/C can also be used. For methylation with methyl iodide cesium carbonate is used as a base which add cost to the process. This base can be replaced with other inorganic or organic base. In patent no. US 9,150,547 B2, Assignee: Hetero Research Foundation has disclosed a commercially viable process for Pazopanib and its salts using novel intermediates (Figure 4).
Figure 4. Synthesis of pazopanib hydrochloride using ethanol, THF, NaHCo3, ethyl acetate.
In this route of synthesis 5-amino-2-methylbenzenesulfonamide (compound 6) is reacted with 2,4-dichloropyrimidine (compound 3) in mixture of ethanol and tetrahydrofuran in the presence of sodium bicarbonate followed by purification in ethyl acetate to get novel compound 5-(4-chloropyrimidin-2-yl-amino)-2-methylbenzenesulfonamide (compound 8) having yield 48%. The other compound N,2,3-trimethyl-2H-indazol-6-amine (compound 7) is prepared wherein 2,3-dimethyl-2H-indazol-6-amine (compound 2) is methylated with paraformaldehyde in the presence of sodium methoxide and sodium borohydride in methanol. Further, these two compounds 7 and 8 are condensed together in ethanol in the presence of hydrochloric acid to afford Pazopanib hydrochloride having yield ~63% and HPLC purity 97.5% which further purified in mixture of methanol (20 V) and water (2 V) to afford pure Pazopanib hydrochloride having HPLC purity 99.9% and yield 77%. The reported yields are considerably low as compared to Innovator’s route of synthesis. In this route during synthesis of compound 8, there is a great possibility for coupling of amino group of compound 6 with second chloro group present in 2,4-dichloropyrimidine to form 5-[(2-chloropyrimidin-4-yl)amino]-2-methylbenzene-1-sulfonamide and same will cause yield loss. In patent application publication no. US 2016/0280689 A1 and patent no. US 10,730,859 B2. Assignee: Laurus labs. Pvt. Ltd., Hyderabad (IN) has disclosed an improved process for Pazopanib and its salts (Figure 5).
Figure 5. Synthesis of pazopanib hydrochloride using acetone, NaOH, mthyliodide, water ethylacetate.
The patent has also disclosed novel polymorphic forms of pazopanib hydrochloride. The route of synthesis is like inventor’s route but there are changes in the reaction solvent, base and reaction temperature to improve yield and quality. In first step, 2,3-dimethyl-2H-indazol-6-amine (compound 2), is reacted with 2,4-dichloropyrimidine (compound 3) in the presence of triethylamine in dimethyl sulfoxide (DMSO) to generate N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine (compound 4). Compound 4 is methylated with methyl iodide in the presence of sodium hydroxide to get N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amine (compound 5) and this further reacted with 5-amino-2-methylbenzenesulfonamide (compound 6) to afford Pazopanib hydrochloride. In this patent, during coupling of compound 2 with compound 3, base is selected from inorganic or organic base except potassium carbonate and in methylation of N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine with methyl iodide, base is selected from inorganic or organic base except potassium carbonate or cesium carbonate. In this patent, in place of cesium carbonate, sodium hydroxide is used which is cost effective at commercial level and same is claimed in the patent. To get the desired quality, Pazopanib hydrochloride is purified by using 80 volume of methanol which make this step unviable at commercial scale. This patent also describes preparation of different polymorphic forms of Pazopanib hydrochloride. A novel route for synthesis of Pazopanib is disclosed in Letters in Organic Chemistry. Volume 9. Issue 4. Pages 276-279. 2012 (Figure 6).
Figure 6. Synthesis of pazopanib using acetic acid.
In this route of synthesis 2-ethyl-5-nitroaniline (compound 9) is cyclized to form 3-methyl-6-nitro-1H-indazole (compound 1) in glacial acetic acid in the presence of aqueous sodium nitrite. 3-methyl-6-nitro-1H-indazole (compound 1) is methylated with trimethyl orthoformate to get 2,3-dimethyl-6-nitro-2H-indazole (compound 10). The nitro group of compound 10 is reduced under hydrogen pressure in the presence of Pd/C and then methylated with paraformaldehyde in the presence of sodium hydride and sodium borohydride to get N,2,3-trimethyl-2H-indazole-6-amine (compound 7). Compound 7 is further reacted with 2,4-dichloropyrimidine (compound 3) in DMF in the presence of sodium bicarbonate to get N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amine (compound 5). Compound 5 is reacted with 5-amino-2-methylbenzenesulfonamide (compound 6) in the presence of HCl in isopropanol to afford Pazopanib hydrochloride. The hydrochloride salt is treated with triethylamine in water to isolate Pazopanib (free base) as a white solid. In CN 107721989 B, Assignee: Suzhou Southeast Pharmaceuticals Co., Ltd., Peop. Rep. China has disclosed a new process for Pazopanib avoiding use of toxic substance i.e., iodomethane, less by-product generation, improved yields, and low production cost (Figure 7).
Figure 7. Synthesis of pazopanib using methanol.
2,3-dimethyl-2H-indazol-6-amine (compound 2) is methylated in the presence of methanol, triphenylphosphine and dichlorocynobenzoquinone in dichloromethane to get N,2,3-trimethyl-2H-indazole-6-amine (compound 7) (yield: 94%). Compound 7 is reacted with 2,4-dichloro-5-nitropyrimidine (compound 11) in the presence of diisopropylethylamine in toluene to get compound 12 (yield: 92%). Compound 12 is reacted with 5-amino-2-methylbenzene sulfonamide (compound 6) in the presence of diisopropylethylamine in methanol to get compound 13 (yield: 87%). The nitro group of compound 13 is reduced under hydrogen pressure in the presence of Pd/C in methanol to get compound 14 (yield: 83%). The compound 14 is further reacted with p-methyl benzenesulfonic acid and nitrous acid special butyl ester in acetonitrile followed by reaction with triethoxy hydrogen silane and (1,5-cyclo-octadiene) ruthenous chloride (II) in dimethylformamide to get Pazopanib which after workup is isolated in ethanol and methyl tert butyl ether having purity more than 99.5% and yield: 83%. The performance of this lengthy route is not evaluated in large scale. The use of palladium to reduce nitro group of compound 13 may deprotect N-methyl group at high hydrogen pressure to form corresponding des-methyl impurity.
In addition to above discussed route of synthesis, other patents are also there where the route of synthesis is like inventor’s route, but some changes have been made in the manufacturing process by changing the reagents used in the process to reduce the overall manufacturing cost of product. In a patent WO 2011/069053 A1 filed by Teva Pharmaceutical Industries Limited, dimethyl carbonate is reported as methylating agent for conversion of compound 4 into compound 5 which is less costly as compared to methyl iodide. In patent US 9,802,923 B2 filed by Sun Pharmaceutical Industries Limited, Mumbai (IN), Raney Nickel is reported for nitro reduction of compound 1 in place of Sn(II)Cl2 used in the base patent.
From the above review, it is clear that Pazopanib can be synthesized in many ways. On comparing reported literature, it can be concluded that the route of synthesis mentioned in Figures 2 and 4 wherein 2,3-dimethyl-2H-indazol-6-amine (compound 2), is reacted with 2,4-dichloropyrimidine (compound 3) to form N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine (compound 4) followed by methylation of secondary amine and coupling with 5-amino-2-methylbenzenesulfonamide (compound 6) to afford Pazopanib hydrochloride is most appropriate procedure. The reported yields of intermediates and Pazopanib (purity more than 99.9%) are considerably good and with slight changes in reaction conditions and volume of solvents the cost of this process can further be reduced. In other route of synthesis, methylation of primary amine is done before coupling with 2,4-dichloropyrimidine (compound 3) and to achieve N-monomethylation of primary amines will remains a fundamental challenge as the starting as well as the product amine are nucleophiles and during reaction the product amine further alkylated to form higher alkylated product. The procedure mentioned in scheme 5 also involves methylation of primary amine and is a lengthy process. This process also uses nitrous acid special butyl ester at later stage of the process which can act as a nitrosating agent to form N-Nitrosamine impurities and therefore, not advisable.
Polymorphic forms of pazopanib
Polymorph of a molecule displays important characteristic property for its solubility, bioavailability, and stability. Pazopanib exhibits polymorphism and several polymorph are developed for pazopanib hydrochloride. Innovator has developed anhydrous crystalline Form-1 for their formulation study and marketed form is also crystalline form-1.
U.S. Patent No. US 7,105,530 discloses a process for preparing pazopanib hydrochloride. As per the European Medicines Agency (EMA) public assessment report innovator’s polymorphic form of Pazopanib hydrochloride is Form-1 (anhydrous). US2012/0197019 A1 and WO 2007/143483 A2 discloses the process for formation of anhydrous crystalline Form-1 of pazopanib hydrochloride from pazopanib hydrochloride hydrate. IPCOM000202288D journal has reported the VOTRIENT® tablet analysis and characterized the crystalline form (Form-1) in the tablet by powder X-ray diffractogram.
There are number of polymorphic forms of pazopanib hydrochloride reported in literature and different claims are reported for different forms. Details of different existing polymorphic forms of pazopanib hydrochloride are tabulated below in Table 4.
| Polymorphic form | Assignee | XRD 2θ values |
Brief detail |
| Form-1 | Laurus Labs Private Limited | 5.3, 10.2, 10.5, 11.4, 13.5, 14.2, 15.3, 16.1, 16.5, 17.7, 19.1, 19.5, 20.1, 20.4, 21.0, 21.9, 23.3, 23.8, 24.0, 25.0, 25.7, 26.1, 26.9, 27.6, 27.9, 28.6, 28.9, 30.3, 32.5, 33.2 and 34.1 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride Form-L8 (20 g) |
| Form-L1 | Laurus Labs Private Limited | 6.4, 7.0, 9.2, 11.5, 12.4, 14.1, 14.3, 15.8, 16.9, 17.8, 18.9, 19.1, 20.4, 20.7, 21.9, 22.8, 23.8, 24.5, 25.4, 26.7, 27.1, 28.0, 28.5, 29.1, 30.8, 31.3, 31.7, 32.0, 32.5, 33.9 and 35.5 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L2 | Laurus Labs Private Limited | 7.4, 7.9, 10.4, 12.5, 13.3, 14.1, 14.8, 15.9, 17.4, 19.0, 21.0, 21.5, 22.6, 23.2, 23.7, 24.2, 25.4, 25.8, 26.2, 26.8, 31.0, 32.3 and 32.6 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L3 | Laurus Labs Private Limited | 7.1, 7.6, 12.0, 13.0, 13.4, 14.4, 15.8, 16.6, 17.7, 18.4, 20.1, 21.4, 22.1, 22.9, 23.7, 24.4, 25.4, 26.4, 26.7, 27.8, 30.5 and 31.3 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L4 | Laurus Labs Private Limited | 7.1, 9.5, 11.5, 13.0, 14.1, 15.0, 16.9, 17.7, 18.1, 19.5, 20.6, 21.6, 22.6, 23.1, 23.7, 24.5, 25.4, 26.3, 26.8, 27.3, 28.7, 30.3, 32.1, 33.2, 34.2 and 35.5 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L5 | Laurus Labs Private Limited | 6.8, 8.9, 9.8, 11.6, 12.9, 14.0, 14.9, 15.4, 17.0, 18.2, 18.8, 19.6, 20.1, 20.5, 20.8, 21.8, 22.6, 23.5, 24.1, 24.6, 25.5, 26.1, 26.7, 27.4, 28.1, 29.4, 30.7, 30.9, 31.4, 32.1, 32.6 and 34.1 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L6 | Laurus Labs Private Limited | 5.5, 6.0, 7.0, 8.4, 9.8, 10.6, 11.1, 12.1, 12.9, 14.1, 15.3, 15.7, 16.2, 16.7, 17.1, 17.9, 18.3, 18.7, 19.1, 20.1, 20.5, 22.7, 23.9, 25.2, 26.3, 28.1, 29.0, 31.6 and 33.5 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L7 | Laurus Labs Private Limited | 5.5, 6.6, 9.6, 10.3, 11.8, 13.6, 14.4, 15.3, 16.2, 16.7, 17.0, 17.7, 19.2, 19.6, 20.2, 20.7, 21.9, 22.4, 23.3, 23.8, 24.1, 24.5, 25.1, 25.8, 26.3, 26.9, 27.3, 27.7, 28.2, 28.8, 29.0, 29.5, 30.4 and 30.9 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (1 g) |
| Form-L8 | Laurus Labs Private Limited | 6.4, 7.9, 10.1, 12.8, 13.2, 13.7, 14.5, 15.1, 16.0, 16.6, 17.0, 17.5, 18.3, 18.6, 19.1, 19.8, 21.3, 22.2, 22.5, 23.2, 23.6, 24.2, 25.1, 25.9, 27.3, 27.8, 29.4, 30.9, 31.5, 34.4 and 36.2 ± 0.2° 2θ. |
Input: Compound 5 (3.0 g) and compound 6 (2.2 g) |
| Form-L9 | Laurus Labs Private Limited | 6.7, 8.7, 9.4, 12.4, 14.4, 14.7, 15.2, 15.8, 17.2, 18.5, 19.5, 20.5, 21.0, 22.0, 23.2, 25.0, 25.5, 26.3, 27.0, 27.5, 29.4, 30.5, 31.4, 32.2, 33.8, 34.9, 35.7, 37.7, 40.2 and 41.5 ± 0.2° 2θ. |
Input: Compound 5 (3.0 g) and compound 6 (2.2 g) |
| Pazopanib Form-A (equivalent to Form-1) | Teva Pharmaceutical Industries Ltd. | 5.6, 10.5, 15.5, 16.4, 16.8, 17.9, 24.0, 24.3, 26.4 and 30.9 ± 0.2° 2θ. |
Input: Compound 5 (48 g) and compound 6 (32 g) |
| Pazopanib di-HCl Form I | Teva Pharmaceutical Industries Ltd. | 6.7, 7.4, 12.0, 13.3, 14.8, 19.0, 23.6, 26.6 and 28.5 ± 0.2° 2θ. |
Input: Pazopanib hydrochloride (200 mg) (Form A) |
| Pazopanib HCl Form II | Teva Pharmaceutical Industries Ltd. | 6.5, 13.0, 15.0, 16.9, 19.0, 19.6, 24.1, 26.3 and 30.1 ±0.2° 2θ. |
Input: Pazopanib hydrochloride (130 mg) (Form A) |
| Pazopanib HCl Form III | Teva Pharmaceutical Industries Ltd. | 9.8, 14.7, 16.7, 18.3, 18.8, 19.8, 24.0, 26.1 and 30.1 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (0.154 g) (Form A) |
| Pazopanib HCl Form IV | Teva Pharmaceutical Industries Ltd. | 11.5, 11.9, 15.5, 17.2, 18.2, 18.7, 21.0, 25.7, 27.1 and 28.6 ± 0.2o 2θ. |
Input: Compound 5 (1 g) and compound 6 (0.67 g) |
| Pazopanib HCl Form V | Teva Pharmaceutical Industries Ltd. | 6.6, 9.4, 12.9, 16.3, 17.4, 19.0, 19.9, 21.3, 21.8, 25.9 and 27.1 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (100 mg) (Form A) |
| Pazopanib HCl Form VI | Teva Pharmaceutical Industries Ltd. | 10.1, 12.7, 14.7, 16.8, 18.2, 19.0, 19.5, 20.0, 22.3, 24.3 and 25.9 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (180 mg) (Form A) |
| Pazopanib HCl Form VIII | Teva Pharmaceutical Industries Ltd. | 10.7, 13.9, 14.6, 15.4, 16.6, 19.7, 23.4, 23.9, 27.6 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (200 mg) (Form A) |
| Pazopanib HCl Form IX | Teva Pharmaceutical Industries Ltd. | 6.2, 12.4, 13.7, 14.6, 16.7, 17.4, 18.3, 19.2, 21.3, 24.0, 25.1 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (200 mg) (Form A) |
| Pazopanib HCl Form X | Teva Pharmaceutical Industries Ltd. | 7.2, 14.5, 17.1, 19.0, 20.9, 23.1, 26.8 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (200 mg) (Form A) |
| Pazopanib HCl Form G | Teva Pharmaceutical Industries Ltd. | 9.6, 11.8, 14.6, 15.3, 16.8, 18.4, 19.6, 20.3, 23.7, 24.7, 26.2 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (171 mg) (Form A) |
| Pazopanib HCl Form XI | Teva Pharmaceutical Industries Ltd. | 6.7, 11.9, 15.1, 15.6, 19.7, 20.9, 24.3, 25.1, 27.3 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (100 mg) (Form A) |
| Pazopanib HCl Form XII | Teva Pharmaceutical Industries Ltd. | 8.9, 9.8, 14.0, 14.4, 17.0, 17.8, 18.9, 19.3, 20.5, 21.5, 26.9 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (0.62 g) (Form A) |
| Pazopanib HCl Form XIII | Teva Pharmaceutical Industries Ltd. | 10.4, 14.4, 15.1, 15.4, 16.6, 19.9, 23.8, 24.5 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (1.04 g) (Form A) |
| Pazopanib di-HCl Form XIV | Teva Pharmaceutical Industries Ltd. | 7.8, 12.4, 14.8, 17.5, 19.0, 22.9, 23.6, 25.3, 26.9, 27.4, 28.5 and 31.7 ± 0.2o 2θ. |
Input: Pazopanib base (0.6 g) |
| Pazopanib di-HCl Form XV | Teva Pharmaceutical Industries Ltd. | 6.9, 12.1, 15.7, 19.4, 23.3, 23.6, 25.7, 26.8, 27.4, 28.5 and 31.7 ± 0.2o 2θ. |
Input: Pazopanib base (0.6 g) |
| Pazopanib Base crystalline Form | Teva Pharmaceutical Industries Ltd. | 7.3, 12.3, 13.2, 13.7, 14.7, 16.1, 18.6, 21.7, 24.7, 26.7 and 28.5 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (1 g) (Form A) |
| Pazopanib di-HCl Polymorph I | Ratio-Parm GMBH | 7.3, 12.3, 13.6, 14.5, 16.0, 18.6, 21.6, 25.8, 26.7 and 28.1 ± 0.2o 2θ. |
Input: Compound 5 (0.2042 g) and compound 6 (0.1289 g) |
| Pazopanib Mono-hydrochloride Polymorph II | Ratio-Parm GMBH | 6.5, 7.8, 9.3, 11.2, 12.8, 13.1, 14.7, 15.6, 18.1, 19.0, 22.5 and 24.0 ± 0.2o 2θ. |
Input: Compound 5 (8.4269 g) and compound 6 (5.9886 g) |
| Pazopanib hydrochloride Form-SP | Shilpa Medicare Ltd. | 6.5, 8.2, 10.3, 11.4, 11.7, 13.1, 17.1, 18.1, 18.5, 20.8 and 23.6 ± 0.2o 2θ. |
Input: Pazopanib hydrochloride (50 g) |
Table 4. Polymorphic forms reported in literature and solvents used for preparation thereof.
In pharmaceutical industry a particular polymorph is treated as a new product. Pazopanib hydrochloride Form 1 is not protected in any patent as product and therefore, it is free to use after expiry of product patent. Other than this, in an Indian application IN5591/CHE/2013 (Assignee: Laurus Labs) covers Form-K1, Form-K2, Form-K3 & Form-K4 of N-(2-chloropyrimidin-4-yl)-N-2,3-trimethyl-2H-indazol-6-amine (compound 5) an intermediate of Pazopanib hydrochloride. As per Biopharmaceutics Classification System (BCS), Pazopanib hydrochloride can be categorized as a class II active substance having low solubility and high permeability. The solubility can be increased by reducing particle size by micronization. The maximum daily dose of Pazopanib is 800 mg and after administration, Pazopanib eliminate slowly with half-life (t1/2) value of ∼31 hours.
Stability of pazopanib hydrochloride
As per CHMP assessment report stability studies were carried out at commercial batches by innovator and samples were kept at 25°C/60% Relative Humidity and 30°C/65% Relative Humidity for 24 months and in 40°C/75% Relative Humidity for 6 months. Samples were analysed for different quality parameters and found to be complying with the defined specification. During stress studies, no significant degradation of product was observed.
Pazopanib hydrochloride is a Tyrosine Kinase Inhibitor (TKI) with potential antineoplastic activity developed and marketed by Novartis/GlaxoSmithKline and was approved by FDA on October 19, 2009. Pazopanib is an oral angiogenesis inhibitor which targets the tyrosine kinase activity associated with Vascular Endothelial Growth Factor Receptor (VEGFR), Platelet-Derived Growth Factor Receptor (PDGFR), and stem cell factor receptor (c-KIT). The maximum recommended daily dose of Pazopanib is 800 mg. The manufacture process reported by innovator consists of mainly four steps by using 3-methyl-6-nitro-1H-indazole (1) as a starting material. There are other alternate route of synthesis having different advantages and thought to have viability at commercial scale have been reported in literature. After review of all data gathered the innovator’s Route of synthesis appears to be most suitable process and with some changes it can further be mode more advantageous such as change of reagents, solvents and their quantity, by doing one-pot synthesis, by avoiding mutagenic reagents etc. There are number of polymorphs reported in literature such as Pazopanib hydrochloride Form-1, L1, L2, L3, L4, L5, L6, L7, L8, L9, Form-A (equivalent to Form-1), Form II, Form III, Form IV, Form V, Form VI, Form VIII, Form IX, Form X, Form XI, Form XII, Form XIII, Form G, Dihydrochloride Form XIV, Dihydrochloride Form XV, Dihydrochloride polymorph I, Dihydrochloride polymorph II, Monohydrochloride Form II, Form SP, Base crystalline form. The stability and advantages of new polymorphic forms other than crystalline Form 1 are not clearly reported in literature. Pazopanib hydrochloride Form 1 have an adequate stability data and during stress studies, no significant degradation in the product has been observed. In future, different derivatives of Pazopanib can be synthesized, and assessed for their inhibitor activity upon series of kinase and can be considered for development of new anticancer drug.
Onkar Chand performed conceptualization, data curation, writing-original draft. Lalita Chopra performed supervision, writing-reviewing, and editing. Shashikant Tiwari performed supervision.
There are no conflicts to declare.
The first author is thankful to the Department of Chemistry, Chandigarh University Gharuan, Mohali, Punjab, and Ind-swift laboratories limited for providing necessary facilities to collect different literature. I would like to thank Lalita Chopra for her continuous guidance for successful compilation of this review article. I would also like to thank Shashi Kant Tiwari for his valuable support and guidance.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]