ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Chandra Bhan Verma1, M.A. Quraishi2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
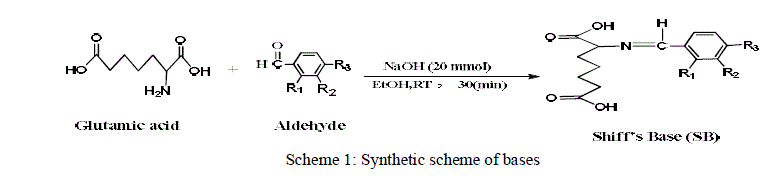
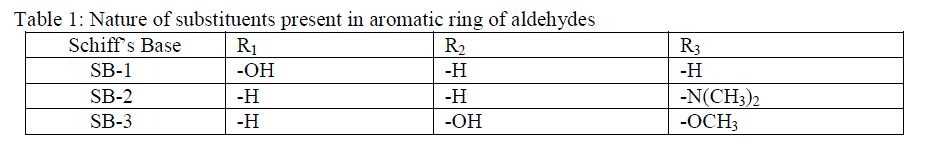
In the present investigation, three Schiff’s bases (SBs) namely 2-(2-hydroxybenzylideneamino) heptanedioic acid (SB-1), 2-(4-(dimethylamino) benzylideneamino) heptanedioic acid (SB-2) and 2-(4-hydroxy-3- methoxybenzylideneamino) heptanedioic acid (SB-3) were synthesized and their corrosion inhibition properties on mild steel in 1M HCl has been investigated using weight loss, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. Potentiodynamic polarization measurements indicate that SBs acts as mixed type corrosion inhibitors. The morphology of the mild steel surface was examined by scanning electron microscopy (SEM), and the surface composition was evaluated using energy-dispersive X-ray spectroscopy (EDX) to show the presence of SBs on the mild steel surface. The adsorption of SBs on the mild steel surface obeys Langmuir adsorption isotherm. Some thermodynamic and kinetic parameters also determined in relevance to describe the mechanism of adsorption
Keywords |
| Schiff’s Base, Acid corrosion, mild steel, thermodynamic parameters, EIS |
INTRODUCTION |
| The use of inhibitors to control the destructive attack of acid environment has found widespread applications in many industrial processes such as acid cleaning, acid pickling, acid descaling, and oil well acidizing [1]. Due to its high mechanical properties and low cost [2], mild steel has a wide application in various industries as construction material for chemical reactors, heat exchange and boiler systems, storage tanks, oil and gas transport pipelines. Organic compounds containing polar functions with oxygen, nitrogen, or sulfur atoms have been widely studied as corrosion inhibitors. Recently some Schiff base reported as effective corrosion inhibitors for mild steel [3-16]. These compounds generally become effective through adsorption on metal surface in which polar groups acting as adsorption centre. The efficient adsorption is the result of either the π-electron of the aromatic system and multiple bonds, or the presence of electronegative atoms (O or N) in the inhibitors molecular structure [17]. Availability of π electron due to the presence of multiple bonds or aromatic rings in the inhibitors molecule would facilitate electron interact with d-orbital of iron [18, 19]. The several Schiff’s bases have been recently investigated as corrosion inhibitor for various metals and alloys [20-22]. The growing attention toward Schiff’s bases as corrosion inhibitor is due to presence of polar –CH= N- bond which act as adsorption center during adsorption of SBs on mild surface in aggressive acid solution [23]. |
| The aim of this paper is to study the inhibiting action of synthesized Schiff bases of Glutamic acid and aldehydes containing nitrogen, oxygen and aromatic rings. The study was conducted by weight-loss, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). Several isotherms were also tested for their relevance to describe the adsorption behavior of investigated SBs. To studied effect of temperature on the corrosion behavior of mild steel, weightloss experiment carried at different temperature in the absence and presence of optimum concentration of SBs. |
II. EXPERIMENTAL |
| 2.1 Inhibitors synthesis |
| The SBs were synthesized according to the scheme 1. All the chemicals and solvents required for the synthesis were purchased from Sigma Aldrich Pvt. Ltd (India). In a typical practice the ethanolic solution aldehydes solution mixed with alkaline ethanolic solution of glutamic acid and stir for 30 minutes. The final mixture is left for about 15 minutes under constant stirring. The completion of reaction was monitored by TLC. The final products were filtered, washed with cold ethanol and dried [24]. The synthesis was also carried using ultrasonic techniques by sonicating the same reaction mixture for 20 min in ultrasonic bath. The purity of the product was confirmed by thin-layer chromatography in ethyl acetate/nhexane (4:6) as developing solvent using the Silica Plate (TLC Plates−Aluminum (Al) Silica. |
 |
 |
| Materials: |
| The mild steel specimens, with composition (wt %) Fe 99.30%, C 0.076%, Si 0.026%, Mn 0.192%, P 0.012%, Cr 0.050%, Ni 0.050%, Al 0.023%, and Cu 0.135%, were abraded successively with emery papers from 600 to 1200 mesh/in grade. The mild steel specimen washed with double distilled water, degreased with acetone and finally dried in hot air blower. The working electrode (WE) was a 7.0 cm long stem (isolated with epoxy resin) to provide an exposed surface area of 1.0 cm2 for electrochemical measurements and dimension 2.5 × 2.0 × 0.025 cm3 were used in weight loss experiments. The test solution 1 M HCl prepared from analytical reagent grade reagent (37 % HCl) and double distilled water. |
| 2.3. Weight loss method |
| The weight loss measurements were carried out by standard method as described earlier [25].The inhibition efficiency (η %) and surface coverage (θ) was calculated by using the following equations: |
 |
| 2.4 Electrochemical measurements |
| The Potentiostat/Galvanostat having a Gamry framework system based on ESA 400 in a frequency range of 10-2 Hz to 105 Hz under Potentiodynamic conditions, with amplitude of 10 mV peak-to-peak, using AC signal at Ecorr was used for all electrochemical measurements. This Potentiostat/Galvanostat consists of three electrode assembly; in which working electrode was mild steel, saturated calomel electrode was reference electrode and platinum foil was counter electrode. Gamry applications include software DC105 for corrosion and EIS 300 for EIS measurements, and Echem Analyst version 5.50 software packages for data fitting. The electrochemical tests have performed in aerated solutions. Prior to the electrochemical measurements the working electrode was immersed in 1 M HCl in absence and presence of SBs for 30 minutes to stabilization of the OCP w.r.t. SCE. All the electrochemical impendence measurements were performed under a potentiodynamic condition from 100,000 Hz to 0.01 Hz with amplitude of 10 mV peak-to-peak. The polarization measurements were performed by changing the electrode potential automatically from -250 to +250mV vs. OCP at a scan rate of 1 mV s-1. The linear Tafel segments of anodic and cathodic curves were extrapolated to the corrosion potential to obtain corrosion current densities (Icorr).. The inhibitive efficiency was calculated by Equations (4) and (5) [26-27]. |
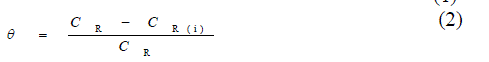 |
| Icorr and Icorr(i) signify the corrosion current density in the absence and presence of SBs; Rct(i) and Rct are charge transfer resistance in the presence and absence of the SBs. |
III. RESULTS AND DISCUSSION |
| 3.1 Weight loss studies: |
| `3.1.1 Effect of inhibitor concentration |
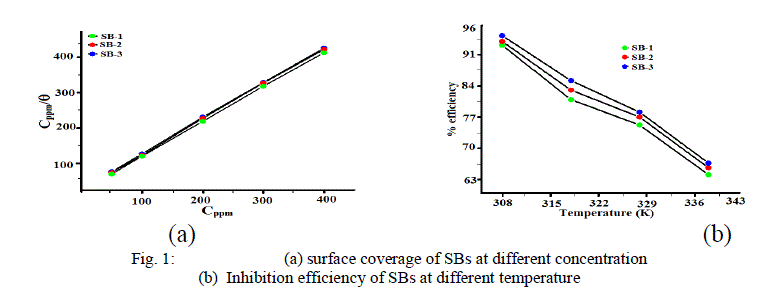
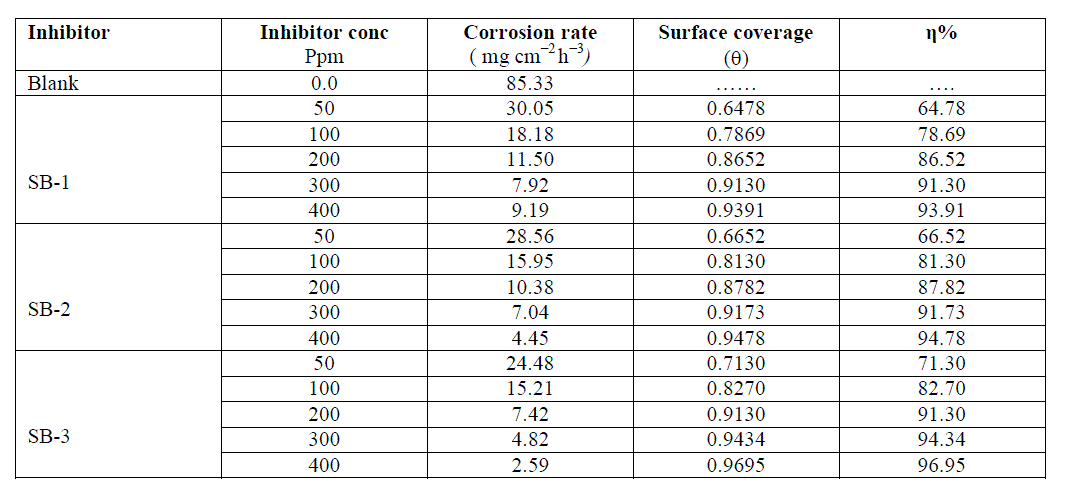
| The values of percentage inhibition efficiency (η %) and corrosion rate (CR) obtained from weight loss method at different concentrations of SBs are given in Table 2. It is observed from the table that inhibition efficiency increase with SBs concentrations. The maximum inhibition efficiency was obtained at 400 ppm and further increase in concentration did not cause any appreciable change in the performance of SBs suggesting that 400 ppm was optimum concentration. The value of corrosion rate decreases and inhibition efficiency increases with the SBs concentrations. This trend may result from the fact that adsorption and surface coverage increased with the increase in SBs concentrations. The variation of inhibition efficiency with increase in inhibitors concentrations is represented in Fig 1(a). From Table 2, it is clear that the order of the inhibition efficiency of SBs is as follows: SB-1 < SB-2 < SB-3. |
 |
| 3.1.2. Effect of temperature |
| The variation of inhibition efficiency with solution temperature is shown in Fig 1(b). It is observed that inhibition efficiency decreases with increase in temperature. The decrease in inhibition efficiency with temperature may be attributed to desorption of adsorb SBs molecules from metal surface at higher temperature [28]. At higher temperature, more desorption of SBs takes place and greater surface area of metal comes in contact with acid environment, resulting in an increase in corrosion rate. These results confirm that all SBs are excellent inhibitors for corrosion of mild steel in 1 M HCl in the range of temperature studied. The excellent performances of studied SBs are due to strong interaction between mild steel surface and SBs molecules. |
| Table 2. Weight loss parameters such as Corrosion rate (CR), Surface coverage (ïÃÂñ) and corr1osion inhibition (ïÃÂè% ) for mild steel in 1M HCl in absence and in presence of different concentrations of SBs derived from weight loss measurements: |
` |
| 3.2.3 Thermodynamic parameters and adsorption isotherm |
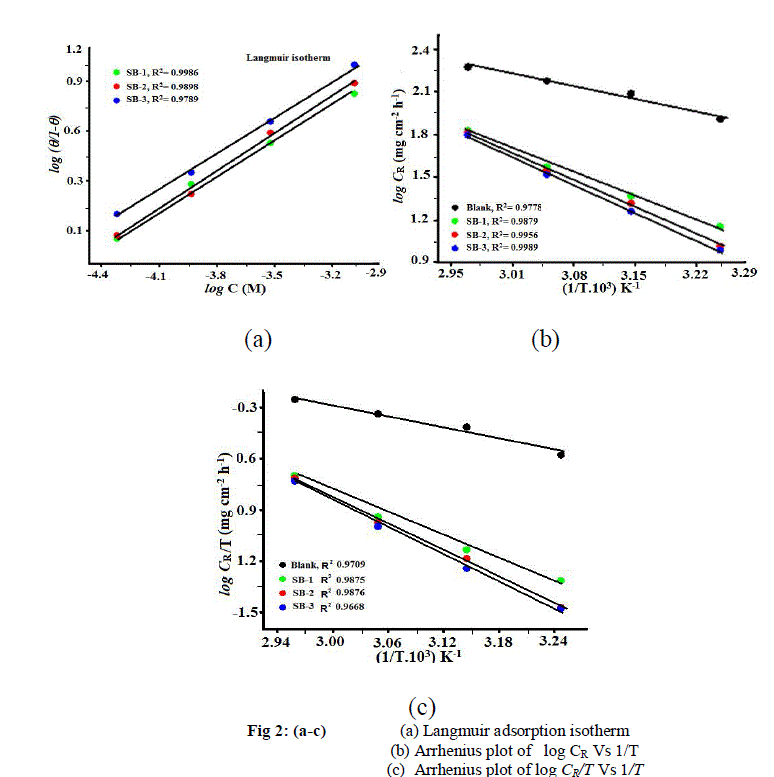
| The mechanism of corrosion inhibition may be explained on basis of adsorption behavior [29]. Several adsorption isotherms were tested to describe the adsorption behavior of SBs, in which best fit was obtained by Langmuir adsorption isotherm. The plot between log C vs logθ(θ) give straight lines for all the SBs as shown in Fig 2 (a). The degree of surface coverage (θ) for different concentrations of all SBs was evaluated from weight loss data. It is found that SBs obey Langmuir adsorption isotherms, which is given by following equation. |
 |
| where Kads is the equilibrium constant of the adsorption–desorption process, is the degree of surface coverage and Cinh is molar concentration of SBs in the bulk solution. Though the Langmuir adsorption isotherm is linear [Fig 2 (a), R2 = > 0.9], the deviation from the ideal behavior (R2 = 1.00) may be due to the molecular interaction among the adsorbed SBs species, a factor which was not taken into consideration during the derivation of the Langmuir equation [30]. Langmuir isotherm assumes that: |
| (i) The metal surface contains a fixed number of adsorption sites and each site holds only one adsorbate.c |
| (ii) ΔGoads is the same for all sites and it is independent of time. |
| (iii) The adsorbates do not interact with one another, i.e. there is no effect of lateral interaction of the adsorbates on ΔGo ads. |
 |
| It is well known that the metallic corrosion inhibition evolves the adsorption of inhibitors molecules on metal surface in the first step which depend upon chemical composition, molecular structure of inhibitor, temperature and the electrochemical potential at the metal/solution interface. In fact, the solvent H2O molecules could also adsorb at metal/solution interface. So the adsorption of organic inhibitors molecules from the aqueous solution can be regarded as a quasi-substitution process between the organic compounds in the aqueous phase [Org(sol)] and water molecules at the electrode surface .[H2O(ads)] [31]. |
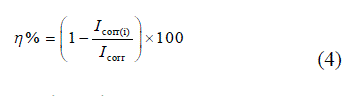 |
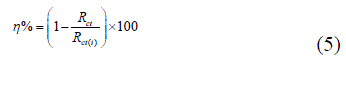 |
| where Ea represent the activation energy for the corrosion of Mild Steel in 1 M HCl, λ pre-exponential factor, R is the gas constant, A the Arrhenius pre-exponential factor and T is the absolute temperature. The plot between log CR vs 1/T (Arrhenius plot) for mild steel in 1M HCl in absence and presence of SBs in 1M HCl solution is depicted in Fig 2 (b). The plots obtained were straight lines and apparent activation energies (Ea) at optimum concentration of SBs were determined by linear regression between log CR vs. 1/T and listed in Table 3. It is clear from the Fig 2 (b) that the linear regression coefficients are close to unity for each SB. The data shows that thermodynamic activation functions (Ea) of the corrosion in mild steel in 1 N HCl solution in the presence of the SBs is higher than those in free acid solution indicating that all the SBs lowers the inhibition efficiency at higher temperature [35-38].The dependency of corrosion rate can also explain by transition stat equation [34]. |
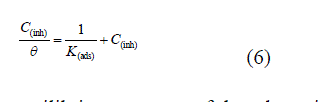 |
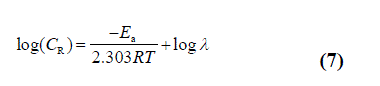 |
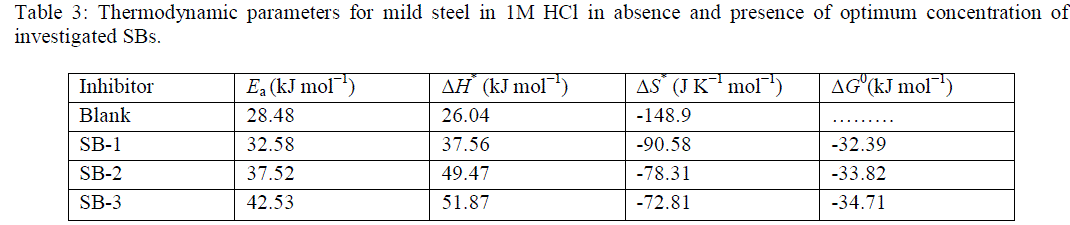 |
| Study of Table 3 reveals that the ΔH* values for dissolution of mild steel in 1M HCl in presence of SBs are higher (37.56– 51.87 kJ mol_1) than that in absence of inhibitors (26.04 kJ mol_1).The positive sign of enthalpy reflect the endothermic nature of mild steel dissolution process meaning that dissolution of mild steel is difficult in presence of SBs as compare in absence of SBs [39]. On comparing the values of entropy of activation (ΔS*) listed in Table 3, it is clear that entropy of activation increased in presence of the studied SBs compared to free acid solution. Such variation is associated with the phenomenon of ordering and disordering of inhibitors molecules on the mild steel surface. The increased entropy of activation in the presence of SBs indicated that disorderness is increased on going from reactant to activated complex on metal/solution interface [40]. |
| The standard free energy of adsorption, (ΔG°ads) at different temperatures were calculated using following formula and given in table 3. |
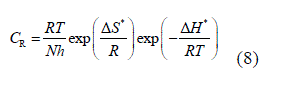 |
| The value 55.5 in this case is the concentration (M) of water in solution [41]. The negative values of ïÃÂÃâGo ads ensure the spontaneity of adsorption process and stability of the adsorbed layer on the mild steel surface. Generally, the values of around -20 kJ mol_1or lower are consistent with physisorption, while those around-40 kJ mol_1 or higher involve chemisorption [42]. |
| 3.2 Electrochemical measurements |
| 3.2.1. Potentiodynamic polarization measurements: |
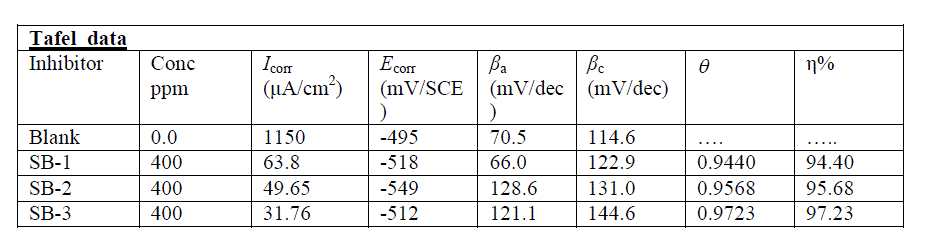
| Polarization measurements were carried out in order to gain knowledge concerning the kinetics of the cathodic and anodic reactions. Fig 3 represents the cathodic and anodic curves for three SBs at optimum concentration in 1M HCl. It could be observed that both the cathodic and anodic reactions were suppressed with the addition of SBs, which suggested that the SBs reduced anodic dissolution and also retarded the hydrogen evolution reaction. The values of electrochemical parameters associated with polarization measurements, such as corrosion potential (Ecorr), corrosion currents densities (Icorr) and Tafel slopes (βa, βc) were calculated by extrapolating the Tafel slope and are listed in Table 4. From table it is clear that inhibition efficiency increases with increasing concentrations and maximum efficiency was obtained was 96% at 400 ppm concentration. |
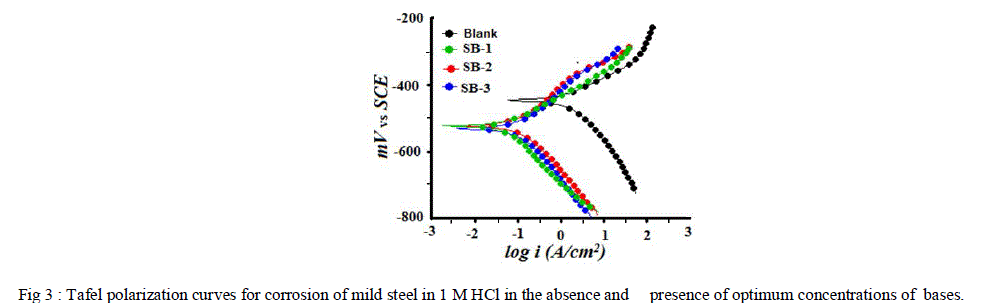 |
| Fig 3 : Tafel polarization curves for corrosion of mild steel in 1 M HCl in the absence and presence of optimum concentrations of bases. |
| According to Ferreira and others [43, 44], if the displacement in (Ecorr) values: |
| (i) >85 mV in inhibited system with respect to uninhibited, the inhibitors could be recognized as cathodic or anodic type and |
| (ii) if displacement in Ecorr is <85 mV, it could be recognized as mixed-type. |
| For studied SBs, the maximum displacement range was 17-54 mV towards cathodic region, which indicates that all studied SBs are mixed-type inhibitors [45]. The values of corrosion current density (Icorr) decreased in presence of SBs which suggests that the rate of electrochemical reaction was reduced due to the formation of a barrier layer over the mild steel surface by the SBs molecule. From Table 4, it is also clear that the values of cathodic and anodic Tafel slope constant are slightly changed in the presence of SBs. This suggest that studied SBs were first adsorbed onto the metal surface and impeded by merely blocking the reaction sites of the metal surface without affecting the anodic and cathodic reaction[46]. Table 4: The Electrochemical Impedance and Linear polarization parameters and corresponding efficiencies of three bases in 1 M HCl at optimum concentration. |
 |
| 3.2.2 Electrochemical Impedance Spectroscopy: |
| Impedance method provides information about the kinetics of the electrode processes and simultaneously about the surface properties of the investigated systems. The shape of impedance gives mechanistic information. The corrosion behavior of mild steel in 1 M HCl in absence an presence of different concentrations of SBs were investigated by EIS after immersion for 30 min at 303 ± 1 K. Nyquist and Bode plots of mild steel in uninhibited and inhibited acid solutions containing optimum concentrations of SBs are presented in Fig 4 (a) and (b). |
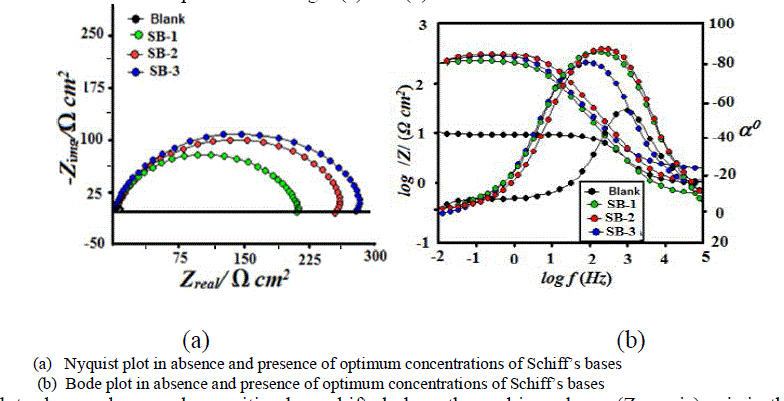 |
| The Nyquist plots show a depressed capacitive loop shifted along the real impedance (Zre axis) axis in the high frequency (HF) range and an inductive loop in the lower frequency (LF) range (in Bode plot). The HF capacitive loop can be attributed to the charge transfer reaction and time constant of the electric double layer and to the surface non-homogeneity of structural or interfacial origin, such as those found in adsorption processes [47]. The LF inductive loop may be attributed to the relaxation of adsorbed compound on electrode surface [48]. It has been observed that diameter of semicircular loop increases with increase SBs concentrations. The increasing diameter of capacitive loop obtained in 1 M HCl in presence of SBs indicated the inhibition of corrosion of mild steel. The EIS parameters for SBs such as Rs, Y0, Rct and Cdl were derived from the Nyquist plot are given in Table 5. |
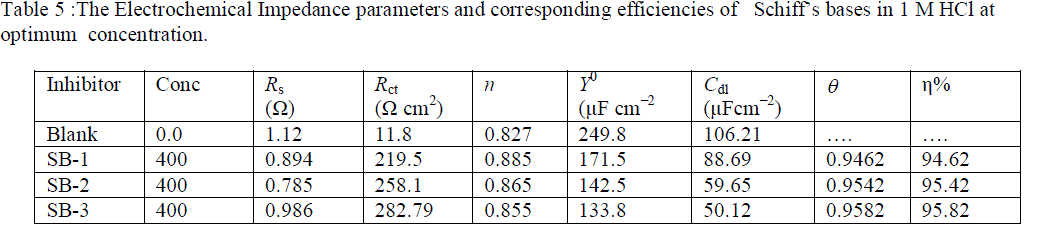 |
| It is apparent from Table 5 that the impedance of the inhibited system amplified with increasing the SBs concentrations and the Cdl values decreased with increasing SBs concentrations. The double layer capacitance (Cdl) was calculated by using following equation [49]: |
| where, Y0 is CPE coefficient, n is CPE exponent (phase shift), ω is the angular frequency. The ωmax represents the frequency at which the imaginary component reaches a maximum. This decrease in Cdl results from a decrease in local dielectric constant and/or an increase in the thickness of the double layer, suggested that SBs molecules inhibit the iron corrosion by adsorption at the metal/acid interface [50]. The depression in Nyquist semicircles is a feature for solid electrodes and often referred to as frequency dispersion and attributed to the roughness and other in homogeneities of the solid electrode [51]. It is worth noting that the percentage inhibition efficiencies obtained from impedance measurements are comparable and run parallel with those obtained from weight loss and potentiodynamic polarization measurements. |
IV. SURFACE INVESTIGATION |
| 4.1. SEM Analysis |
| Fig 5 (a-d), represent the morphology of the mild steel specimens immersed in 1M HCl in absence and presence of optimum concentration of the SBs for 3 h immersion time. The surface morphology of the uninhibited mild steed is shown in Fig 5 (a) which is characterized by a very rough surface with cracks and pits due to rapid corrosion attack; it can be concluded that mild steel surface was greatly damaged in absence of SBs. The morphology of the inhibited mild steel specimens were shown in Fig (b-d), from Fig it can be concluded that the mild steel surface morphology is remarkably improved in presence of SBs. This finding further suggests the formation of protective film of SBs on mild steel surface. |
 |
| 4.2. EDX Analysis |
| The aim of this study was to conform the finding of weight loss and electrochemical measurements that SBs form a protective film on mild steel surface. To achieve this target, the EDX spectra of mild steel was recorded in 1M HCl in absence and presence of optimum concentration of SBs. |
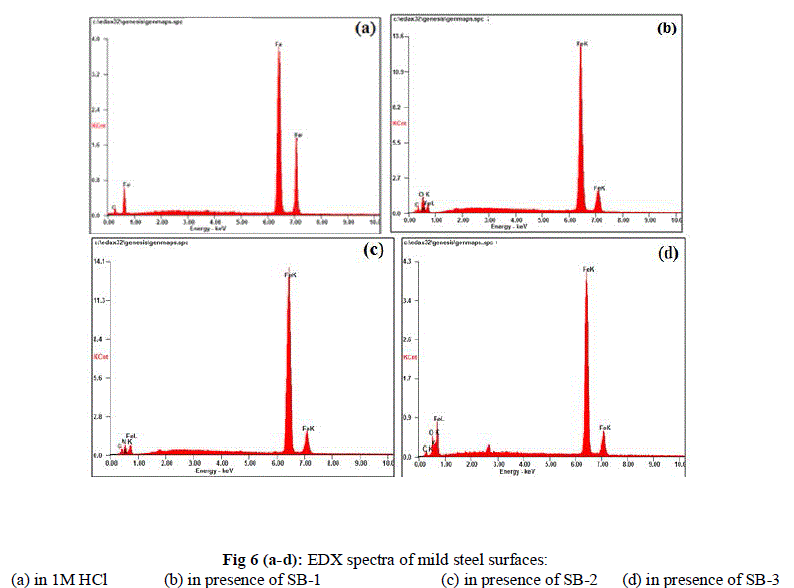 |
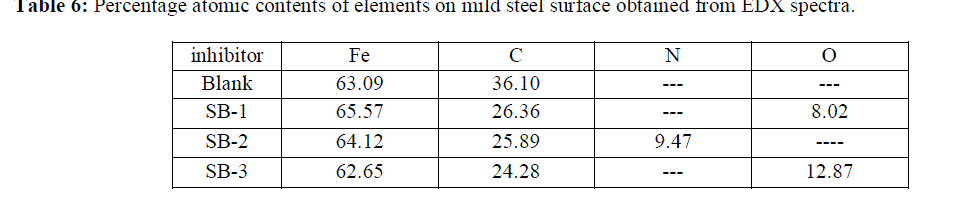
| The EDX spectra of mild steel in absence and presence of SBs is given in Fig 5 (a-d) and the percentage atomic content obtained by EDX spectra is given in table 6. Fig 5 (a) represent the EDX spectra of uninhibited mild steel which peaks for O and N were absent. However the EDX spectra of mild steel in presence of SBs shows characteristic peaks for Oxygen and nitrogen, which suggest that SBs form a protective film on mild steel surface. |
 |
V. MECHANISM OF INHIBITION |
| Corrosion inhibition of mild steel in 1M HCl by SBs can be explained on the basis of molecular adsorption of SBs on to the mild steel surface. It is generally considered that the first step in the corrosion inhibition of a metal is the adsorption of the SBs molecules at metal / solution interface [52]. |
| Thus SBs can adsorb on the mild steel surface by following ways: |
| (a) Electrostatic interaction between the charged molecules and charged metal; |
| (b) Interaction of π-electrons with the metal; |
| (c) Interaction of unshared pair of electrons in the molecule with the metal; and |
| (d) The combination of the all the effects 53-55]. |
| Concerning inhibitors, the inhibition efficiency depends on several factors; such as the number of adsorption sites and their charge density, molecular size, heat of hydrogenation, mode of interaction with the metal surface and the formation metallic complexes. The order of inhibition efficiency of SBs is as follows: |
| SB-3 > SB-2 > SB-1 |
| The adsorption of organic inhibitor cannot be considers as purely physical or purely chemical phenomenon. The adsorption mechanism is influenced by nature and charge on metal surface and chemical structure of inhibitors. The charge on metal surface is due to electric field which emerges at the metal/electrolyte interface. It is well-known that mild steel specimens are positively charged with respect to the potential of zero charge (PZC) in acid solutions It is a well-known fact that the inhibitors not only offer electrons to metal atoms but also have unoccupied higher energy orbital to accept electrons from dorbital of metal atom for strengthening of bonding interaction [56, 57]. A schematic illustration of different modes of adsorption of SBs on metal/acid interface is shown in Fig 5. |
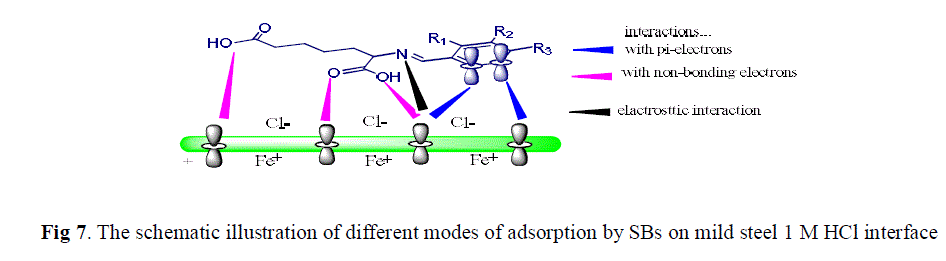 |
| In aqueous solution of 1M HCl SBs molecules may adsorb through protonated heteroatoms and already adsorbed Cl- on mild steel surface. Initially, the protonated form of SBs molecules in acid medium start competing with H+ ions for electrons on mild steel surface. After release of H2 gas, the cationic form of inhibitors returns to its neutral form and heteroatoms with free lone pair electrons promote chemical adsorption. Thus, there is a synergism between the adsorbed Cl− ions and protonated SBs. Hence, we can assume that the inhibition of mild steel corrosion in 1 M HCl is due to the adsorption of SBs on the mild steel surface. |
VI. CONCLUSIONS |
| From above study it is concluded that: |
| 1. SBs are good corrosion inhibitors for corrosion of mild steel in 1M HCl solution. The maximum efficiency was found to be 97% at 400 ppm concentration. |
| 2. The adsorption of SBs molecule on mild steel surface obeyed the Langmuir isotherm. |
| 3. The Potentiodynamic studies reveal that SBs are mixed type inhibitors i.e. it affected both cathodic and anodic reactions. |
| 4. The negative values of ΔG shows that adsorption of inhibitors on mild steel is a spontaneous process. |
| 5. The results obtained from weight loss and electrochemical methods are in good agreement. |
References |
|