ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Saman Mushir1, Satyanarayan Deep2, Tasneem Fatma3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Cyanobacteria has evolved the capacity to synthesize, mount up and metabolize scytonemin a photoprotective pigment as a part of an overall tactic to taper the unswerving and oblique destructive effects of environmental ultraviolet radiation (UVR) due to the deterioration of ozone layer attributable to the release of pollution containing the chemicals chlorine and bromine. Scytonemin is an indole phenolic pigment found in the sheath of many cyanobacteria having a unique dimeric structure, ecological importance and novel pharmaceutical activity have enthused substantial pursuit in its biosynthesis. This study includes the screening of scytonemin from 46 studied cyanobacterial strains out of which 23 showed the presence of scytonemin. Aulosira fertilissima showed the maximum scytonemin. The effect of environmental factors, including Light intensity, photoperiod, UV-light was studied on scytonemin synthesis of A. fertilissima. A remarkable change in scytonemin synthesis was observed under UV-light stress. Scytonemin increased under all stress conditions but it increased maximally under UV-light stress
Keywords |
| Aulosira fertilissima, absorption spectrum, Cyanobacteria, photoprotective pigment, environmental stress. |
INTRODUCTION |
| Diminution in stratospheric ozone levels will initiate to copious amount detrimental ultraviolet radiations accomplish the Earth's surface which results of which Morphology, cell differentiation, survival, growth, pigmentation, motility and orientation, N2 metabolism, phycobiliprotein composition, protein profile, DNA and 14CO2 uptake have been reported to be affected by ultraviolet radiations [1, 2, 3]. The highly vigorous ultraviolet rays (UV-B; 290–320 nm) has the paramount potential for cell damage caused by both direct effects on DNA, proteins and devious effects via the production of reactive oxygen species [4, 5]. One of the adaptations whereby cyanobacteria can thwart the mutilation persuaded by ultraviolet radiations is the synthesis of UV-absorbing/screening scytonemin. Shibata (1969) [6]reported finding of UV absorbing substances in water extracts from several species of corals and a cyanobacterium (likely Trichodesmium) from the Great Barrier Reef. Shibata speculated that these compounds (named S-320) must have a biological function such as UV-photoprotection. Scytonemin (mw544 Da), a dimer of indolic and phenolic subunits, first reported in some terrestrial cyanobacteria is a yellow–brown lipid soluble pigment located in the extracellular polysaccharide sheath of about 300 cyanobacterial species. Scytonemin are highly stable found to act as ultraviolet sunscreen in cyanobacteria, with the greatest absorption in the spectral range of UV-A and therefore may have application in sunscreens. The high concentration of scytonemin in many cyanobacterial sheaths might be providing significant protection to other microorganisms living within and beneath the upper layer of sheathed cyanobacteria. In addition to the important role of scytonemin in cyanobacterial adaptation, this pigment certainly play a vital role in microbial communities exposed to high solar radiation [7]. Scytonemin has also been identified and characterized as an antiproliferative pharmacophore that inhibits cell cycle kinases [8]. The presence of scytonemin in cyanobacterial sheath has been reported to reduce the entry of UV-A radiation in the cell by 90% [7]. The evidence for the photoprotective role of scytonemin has been shown in a number of cyanobacteria from various harsh habitats. Effect of bright light in promoting scytonemin synthesis was also reported [9]. Recently, scytonemin and its derivatives having absorption maxima in both UV-B and UV-A regions have received much attention for their putative role as UVscreening/ absorbing compounds as well as their pharmacological potentials. This study is done to screen the highest scytonemin yielding cyanobacterium and to see the effect of various environmental perspectives on scytonemin in the selected strain. This study provides the environmental factors under which high scytonemin is observed. |
II. MATERIALS AND METHODS |
| A. Strains and growth conditions The strains used in the study were procured from Centre for Utilization and Conservation of Blue Green Algae, Indian Institute of Agriculture and Research Institute New Delhi, India and were maintained in BG11 media [10], except Spirulina platensis which was grown in CFTRI medium [11]. Cultures were grown in BOD at 30 ± 1ºC in 12:12 light: Dark period and 25 μmol m-2 s-1 illuminations. Screening was followed by finding out effect of different environmental factors (Light intensity, photoperiod and UV-light) for the yield of scytonemin yield. B. Extraction and measurement of Scytonemin For extraction of scytonemin samples of harvested cyanobacterial biomass were dried and grounded with mortar and pestle. Cells were suspended in 100% acetone and kept overnight at 4ºC. After centrifugation sample is filtered through whatman filter papers number 1 and absorbance specific filtered contents were measured on UV-visible spectrophotometer. Absorbance of filtrate was measured at 384 nm (scytonemin maximum), 490 nm (pooled carotenoid), 663 nm (chl. a). The value of absorbance at 750 nm was subtracted from all measured absorbance. The cellular scytonemin content was calculated using the trichromatic equation. A*384 (Scyt) = 1.04A384 - 0.79A663 – 0.27A490 Aλ is the measured absorbance at λ. In the absence of absorption coefficient for scytonemin, the unit represent the absorbance of 1mg dry weight of material extracted in 1 ml acetone in a cuvette with 1 cm path length. Specific content are expressed as (Aλ . mg-1). C. Effect of various environmental stresses on scytonemin in Aulosira fertilissima. The effect of temperature was examined by raising the cells of A. fertilissima strain at different experimental light intensity at different region of spectrum viz. light intensity (12.5, 25, 36.5 and 50 μmol photons/m2/s ), photoperiod (00:24, 08:16, 10:14, 14:10, 16:08 and 24:00 L/D) and UV-light [00 min(control), 4 min, 8 min, 12 min and 16 min maintained in BOD cabinet. D. Statistical analysis All analysis was conducted using Graphpad Prism version-5.0 (Graph Pad software, SanDiego, CA, USA). Statistical analysis of three replicates was done by one way analysis of variance (ANNOVA). Dunnett‘s multiple comparison test was used in experimental setup with control in which significant difference at a level of significance of 0.01, 0.001 and 0.0001 (p<0.01, p<0.001, p<0.0001) and âÃâ¬Ãâns‘ for non significant are represented, while post tests for linear trend method was used in experiment setup without control. |
III. EXPERIMENTAL RESULTS |
 |
 |
 |
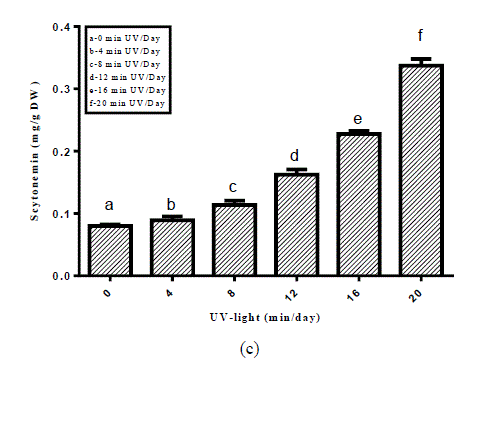 |
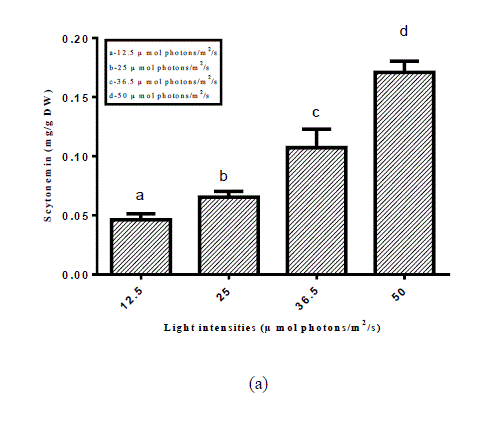
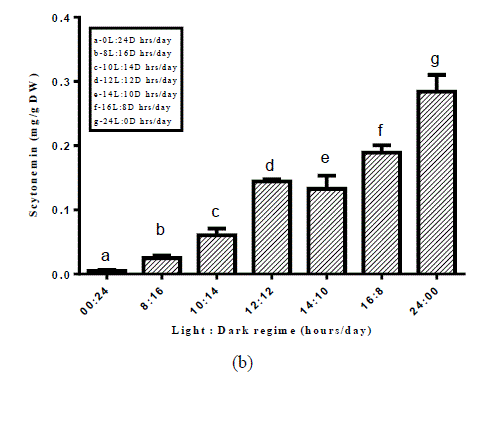
| Fig. 1. Scytonemin under various environmental stress factors (a) light intensities (b) light and dark regime (c) UV-light stress |
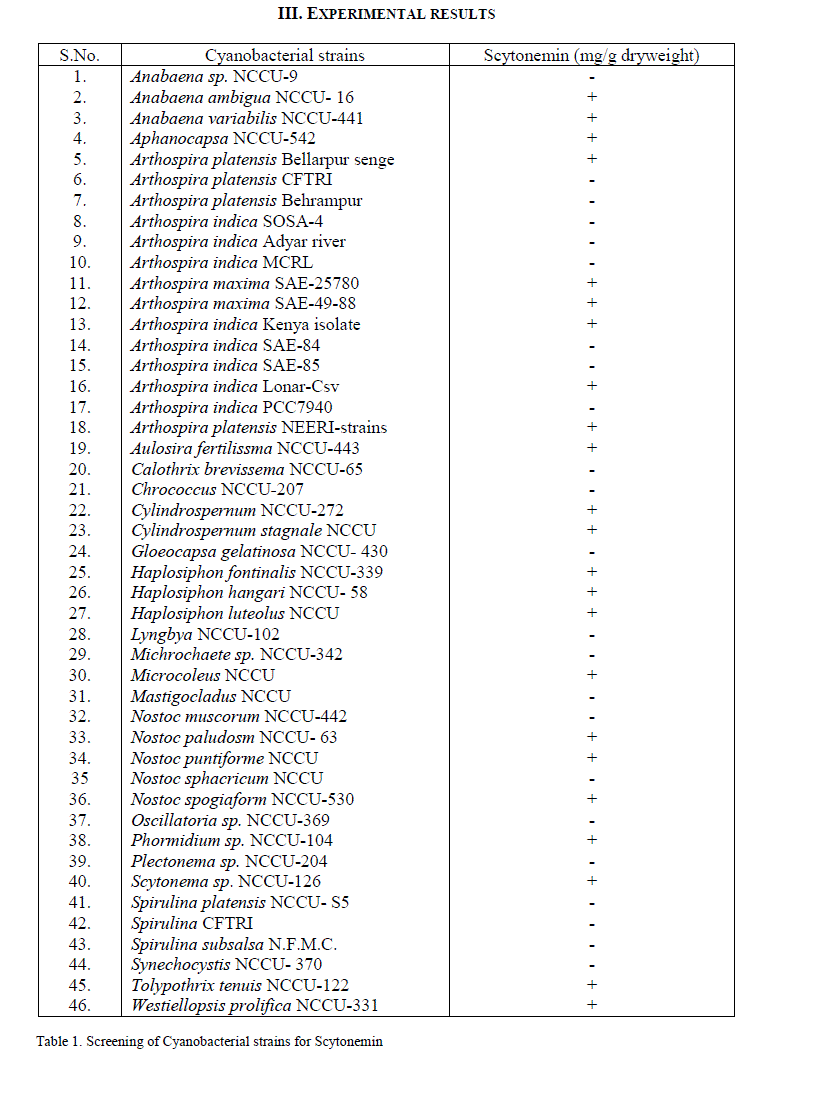
| Among 46 screened cyanobacterial strains scytonemin could be detected in 23 cyanobacterial strains (Table 1). Maximum amount of scytonemin was found in Aulosira fertilissima. Arthospira indica (Lonar-Csv) was the least scytonemin containing cyanobacterial strain. In scytonemin the least amount containing strain was non-heterocystous. As a result of screening amongst 46 strains 23 strains showed the presence of scytonemin in which A.fertilissima was found to be the best candidate and hence used for enhancing the yield of scytonemin by alterations in physicochemical environmental factors like light intensity, Light: Dark regime and ultraviolet light. For enhancing the yield of scytonemin of A.fertilissima alterations in physicochemical growth conditions were attempted. Light intensity less than control (50 μ mol photons/m2/s) showed reduction in this photoprotective pigment. Reduction in scytonemin was higher at lower light intensities (Fig.1). Scytonemin showed correlation with light intensity in A.fertilissima. Light intensity less than control (50 μ mol photons/m2/s) showed reduction in it. Garcia- Pichel et al., (1992) reported in cyanobacterium Chlorogloeopsis sp. 0.05 μg.mg[DW]-1 scytonemin was observed at 50 μ mol m-2s-1 that was increased to 11.51 μg.mg[DW]-1 when light intensity was increased to 200 μ mol m-2s-1 i.e. increased light intensity stress also resulted in scytonemin increase. We could not set experiment above 50 μ mol m-2s-1 light intensity due to limitation of the BOD cabinet light source. This increase in scytonemin may be due to increase in the synthesis of aromatic amino acids metabolites needed in scytonemin synthesis at high photon fluence rate [12]. Light and dark period of a day also imparted their role in scytonemin content of A.fertilissima. Scytonemin showed increase during complete light period (24:00 Light: Dark) as compared to complete darkness (00:24 Light: Dark), the increase in scytonemin was approximately two times higher than control (12:12 Light: Dark) (Fig.2). In light and dark regime scytonemin was negligible in complete 24 hrs dark period. We observed that bright light promots scytonemin synthesis which was also reported by Garcia-Pichel and Castenholz, 1991. Ultraviolet light induced higher synthesis of scytonemin and was highly increase at 20 min/day UV-light exposure (Fig. 3). This increase was approximately three times as compared to the control. Scytonemin increased in A. fertilissima with increasing duration of UV-light exposure. Maximal scytonemin was observed at 20 min/day. It is well established that scytonemin synthesis is primarily induced by UV-A radiation and is highly stable against strong UV radiation [12, 13]. The high amount of scytonemin is required for uninhibited photosynthesis under high UV fluxes in a monospecific population of Calothrix sp. [14]. In Nostoc commune higher far-UV-A irradiations led to only very low scytonemin production whereas in presence of near-UV-A irradiation scytonemin synthesis increased to two- to threefold in comparison to cultures treated with UV-B hence they suggested that the synthesis of scytonemin is triggered by different UV photoreceptors [15]. Similarly Garcia-Pichel and Belnap, (1996) [16] reported that UV exposure is essential for scytonemin synthesis. Pentecost, (1993) [17] has examined a correlation between UV flux and scytonemin content in population of Scytonema and Rivularia sp. and found a negative correlation between UV and scytonemin in Scytonema, but a positive correlation in Rivularia. In the terrestrial cyanobacterium Chlorogloeopsis sp., it has been reported that UV-A exposure induced photoinhibition and growth delay until substantial concentrations of scytonernin had been synthesized and deposited in the extracellular envelopes [18]. Scytonemin showed stability during the stress conditions. |
IV. CONCLUSION |
| The UV-protective pigment scytonemin was present in a few cyanobacterial strains, and its content can be manipulated by altering culture conditions. In the present study, among the screened strains, A. fertilissima yielded maximum scytonemin. 24 hrs light exposure, UV exposure (16 min/day) had enhanced scytonemin yield maximally. Scytonemin is a promising photoprotective pigment with number of pharmacological potentials. |
References |
|