ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Gopinath S.M, Vidya G.M*, Suneetha.T.B, Ismail Shareef.M, Narasimha Murthy T.P, Ashalatha.
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Many compounds of plant origin that inhibit HIV during various stages of cycle, which includes several Bioactive compounds like alkaloids, flavonoids, coumarins, anthroquinones, quinones, steroids, tannins, terpenoids, phenolic compounds, catechin and saponins. These compounds have their own potential to come up as a drug for the treatment of HIV infection. So the aim is to identify the principle activity against HIV virus. These natural productspossessing anti-HIV activity has been summarized with recent outcomes from natural sources has anti-HIV agents
Keywords |
| Anti-HIV, Bio-active compounds, medicinal plants, Drug. |
INTRODUCTION |
| The Human Immunodeficiency Virus (HIV-1), is a one of the members of the retrovirus family, has been a causative organism in an Acquired Immunodeficiency Syndrome (AIDS). The human immunodeficiency virus (HIV) results in lifethreatening opportunistic infections and malignancies. HIV virus destroying the body’s ability to fight against infections, and reduce the functional impairment and damage the immune system, Infection with HIV occurs by the transfer of blood, semen, vaginal fluid, pre-ejaculate, or breast milk. Within these bodily fluids[1].Acquired Immunodeficiency Syndrome (AIDS) that has formed a major health care problem not only in India but also globally. It is a serious disease spreading rapidly in every country. This is an estimated 35.3 million people were living with HIV in 2012. At the same time the number of AIDS deaths also fading in the coming years. Although significant changes remain [2]. |
| Most of the anti-HIV compounds can be assigned to one of these ten classes of HIV inhibitors, according to the stage at which they interfere with the HIV replication cycle, e.g., Adsorption, fusion, uncoating, reverse transcription, integration, DNA replication, transcription, translation, maturation and budding (assembly/release). Many plant products are being used by patients with AIDS in some countries without any scientific proof that they possess anti-HIV activity. Traditional healers are now offering their remedies for scientific evaluation, and a number of studies provide information on the inhibitory activity against HIV of selected plants. Many of the plant derived substances that showed the anti-HIV activity, e.g., alkaloids, flavonoids, coumarins, tannins, catechin, steroids and terpenoids, and tannins [3]. |
| Viruses are intracellular parasites that includes cellular processes, such as nuclear import and export machineries, to perform virus specific functions. For this purpose, many viral proteins and nucleoprotein complexes shuttle between the nuclear and cytoplasmic compartments, and this process is especially critical for viruses with a distinctapplication step occurring in the nuleus. |
| Among many different families of these viruses, retrovirus and lentiviruses in particular, are of major because of their clinical importance (HIV-1 is the most notable lentiviral pathogenic agent) and their potential relevance as vectors for genetherapy.While replication in the host cell’s nucleus provides clear benefits for the virus, such as ready access to cellular transcription and splicing apparatus, it |
| imposes the barrier of the nuclear envelope that has to be overcome. During the early (preintegration) stages of infection, lentiviruses have to transport their genome from the site of penetration to the nucleus and then through the nuclear membrane, while at the later (postintegration) stages they need to import regulatory proteins (Tat and Rev in the case of HIV-1) to stimulate transcription and regulate splicing and nuclear export of subgenomic and genomic RNAs. Given such important role that nuclear import plays in HIV-1 life cycle, it presents an attractive target for antiviral therapeutic intervention [4]. |
| Non-Nuceotide Reverse Transcriptase Inhibitors (NNRTIs) are the group of compounds that inhibit the activity of reverse Treanscriptase Type-1 enzyme of HIV-1 by the virtue of their ability to bind irreversibly at the non-substrate binding, allosteric site.Plant derived compounds have also played an important role in the development of several clinically useful anticancer agents, which regulate cancer related gene expression, induction of apoptosis, cell cycle arrest/DNA fragmentation and inhibition of different cellular enzymes.The current strategy for the treatment of HIV infection is Highly Active Antiretroviral Therapy (HAART), This is based on the combination of inhibitirs of reverse transcriptase and protease, which reduces the death from AIDS related diseases [5]. |
| The HAART treatment is currently available in the industry. It reduces the burden of diseases, but it is associated with various side effects and the necessity for long-term anti-HIV treatments are the limits to standard HIV therapy, Therefore, it is better to discover novel anti-HIV agents from natural sources that may have lesser side effects, better tolerated, less expensive and freely available. The plant extracts or purified phytochemicals may exhibit anti-HIV activity by inhibiting virus entry/fusion and further to prevent sexual transmission of HIV. One of the import enzyme necessary for the replication of this virus is HIV-protease. It belongs to the family, expertlyprotease, it includes a dimer 99 amino acids each. This enzyme plays a crucial role in the process of viral maturation and infectivity.Thus, there is a need for the discovery of the therapeutic strategies. One of the strategies has been to identify anti-HIV compounds from natural sources, particularly from plants and animal origins [6]. |
II. REVIEW OF LITERATURE |
| The traditional medicinal plants can be used because of a persistent and urgent need for anti-HIV drugs to the people. Because the traditional plants are relatively low cost, these plants have been increasingly explored for production of biomedicine and vaccine. Numerous plant derived substance, includingphenylcoumarins and plant proteins have shown good anti-HIV activity that can be related to inhibition of major HIV enzymes such as Reverse Transcriptase (RT) and protease. Plant products have attracted attention as possible anti-HIV drugs targeted on the different steps of the viral life cycle, such as viral attachment and entry and an essential enzymes that play a role during vial genome transcription. The virus infects CD4+ T lymphocytes and macrophages and its genetic material is integrated into the infected cell genome. Several plant extracts have been shown to possess activity against HIV by inhibiting various viral enzymes, and also medicinal plants as potential sources of new active agents not only combine the advantage of being relatively non-toxic and hence more tolerable than rationally designed drugs, but also represent an affordable and valuable source of pharmacologically active substances that can be made sufficiently available through cultivation [7].The actual activity in host cell, the viral RNA is reverse transcribed by Reverse Transcriptase (RT). This viral DNA, then integrated in the host genetic material with the help of the integrase enzyme. |
| And the third most important HIV-1 protease plays an important role in promoting virus maturation and thus promoting infection of new cells. The inhibitors may target in the many stages of the life cycle: virus adsorption, virus cell fusion, virus uncoating, HIV regulatory proteins and HIV enzymes (Reverse Transcriptase, Integrase and Protease) [8]. |
| Andrographis paniculata is an annual herbaceous plant in the family Acanthaceae, native to India and Sri Lanka. It is widely cultivated in Southern and Southeastern Asia. It is commonly known as the king of bitters, various vernacular names are Maha-tita, Bhui-num which means the name of the ground. Mainly this A.paniculatabelongs to Neem tree. As an Ayurvedaherb, it is known as Kalmegh or Kalamegha, this means “dark cloud”. Andrographispaniculata grows at a height of 30-110 cm in shady places with glabrous leaves and white flowers, stem is in the dark green, which grows in height 2-6 mm in diameter, and it is having a slightly enlarged node: leaves upto8. 0 cm long and 2.5 cm broad, seedsnumerals that are in sub quadrate and yellowish brown, mainly leaves and roots used for the medicinal purpose. Active bioactive compounds present in this are Lactones and flavonoids. The primary medicinal compound present in this is Andrographide, this is one of the main compounds which helps to prevent and to treat many diseases. Mainly four Lactones are there they are: deoxyandrographide, andrographide, neoandrographide and 14-deoxy-11,12didehydroandrographide. In the same way Flavonoids contains isoandrographide, homoandrographide, andrographan, andrographosterin and stigmosterol. Pharmacokinetics studies showed that andrographide is quickly absorbed and extensively metabolized in rats and humans. The areal part of the plant is used to extract the active bioactive compounds. A. Paniculata has been reported as having antibacterial, antimalarial, antifungal, antiviral, antiinflamatory, fertility effects and protection of the liver and gallbladder. And also in broad range of pharmacological effects. The main activity of this plant is that it willpufifiesa bloodwhen it is entered into the human body, so that it cures Leprosy, Gonorrhea, Seasonal fevers, and also helpsin the prevention and treatment of the common cold. And it is extremely used as a hepatoprotective agent in Indian system of medicine [9]. |
| Cinnemomum verum also called as true cinnemon, Ceylon cinnemon or Sri Lanka cinnemon, it is a small evergreen tree belonging to the family Lauraceae, native to Sri Lanka. Among other spices, its inner bark is used to make cinnemon. |
| Cinnemon is a common spice used by different cultures, most commonly inner bark of the tree is used for the medical purpose of the family Lauraceae. In Ayurvedicmedicine, it is considered as the remedy for digestive, respiratory and gynecological problems. Volatile oil is produced from the composition of bark, leaf and root bark, so that it might be vary from pharmacological effects. It produces a Hydrocarbons property by primary constituents from different parts of the plant that is cinnemaldehyde from bark, eugenol from leaf and camphor by root. C. verum tree grows around 10 m and leathery leaves, usually opposite side in 11-16 cm long with pointed tips, flowers are in yellow colour which are tubular with 6 lobes, grow in panicles that are as long as the leaves. The fruit is a small, fleshy berry, 1-1.5 cm long that ripens to black. Ti produces three types of essential oils as well as it helps for the digestive system. |
| Cinnemon verum gives numerous beneficial health effects against the HIV activity, and this plant exhibits antiinflammatory property, anti-microbial activity, reducing cardiovascular disease, boosting cognitive function and reducing risk of colonic cancer.Recent studies suggest that consumption of cinnemon on a daily basis could significantly lower blood sugar and cholesterol levels and making it extremely helpful for patients suffering from Type 2 diabetes. Cinnemon essential oil has significant antioxidant and antimicrobial properties as well [10]. |
III. MATERIALS AND METHODS |
| Collection of the material |
| Procurement of raw plant material |
| Leaves and stem barks of the Andrographispaniculata and Cinnemonverum plants were washed in distilled water, chopped into small pieces and allowed to dry at room temperature in the shade for at least two weeks. Dried material was ground to powder. |
| Preparation of extracts |
| Soxhtet Extraction |
| The plant material (50g) was first extracted in soxhlet apparatus with Methanol, Ethanol and Water (350-500 ml) at 35- 40ºC respectively, for 2-3 days. The extract was evaporated to dryness until it becomes 5-10 ml and stored in airtight bottles for further use. |
| Magnetic stirrer Extraction |
| The plant material (50 g) was extracted in Magnetic stirrer with Petroleum ether, n-Hexane and with the Chloroform about 150-200 ml of respective solvents at room temperature for 4-6 hours. This was filtered through Whatman filter paper No.3, the filtrate was evaporated to dryness until it becomes 5-10 ml and stored in airtight bottles for further use. |
| Chemicals |
| Ethanol, Methanol, Water, Petroleum Ether, n-Hexane, Chloroform, con. HCL, Mayers reagent, Magnetiumtunning, Alcoholic KOH, 2% gelatin, Neutral ferric chloride, Magnetium acetate solution, Sulphuric acid, acetic anhydride, Ehrlish reagent, Hemoglobin powder, pepsin and 5% TCA. |
| Phytochemical analysis |
| Each extract was tested for the presence of the phytochemicals was carried out for Alkaloids, Coumarins, tannins, Phenolic compounds, Anthraquinones, Quinones, Steroids and Terpenoids, Catechin, saponines. |
| Phytochemical screening of the plant |
| Detection of alkaloids |
| Mayers test: Filtrates were treated with Mayer’s reagent (potassium Mercuric Iodide). Formation of yellow colored precipitate indicates the presence of alkaloids. |
| Wagner’s Test: Filtrates were treated with Wagner’s reagent (Iodine in potassium Iodide). The formation of a brown / reddish precipitate indicates the presence of alkaloids. |
| Dragendroff’s Test: Filtrates were treated with Dragendroff’s reagent (solution of potassium bismuth iodide). Formation of a red precipitate indicates the presence of alkaloids. |
| Hager’s Test: Filtrates were treated with Hager’s reagent (saturated picric acid solution). Presence of alkaloids confirmed by the formation of a yellow colored precipitate. |
| Detection of flavonoids |
| Alkine reagent test: Extracts were treated with a few drops of sodium hydroxide solution, formation of intense yellow colour, which becomes colorless on the addition of dilute acid, indicates the presence of flavonoids. |
| Lead acetate Test: Extracts were treated with a few drops of lead acetate solution. Formation of a yellow colour precipitate indicates the presence of flavonoids. |
| Detection of coumarins |
| 10 mg of the extract dissolved in methanol and alcoholic KOH was added. And yellow colourdecolorizes while adding con. Hcl, this shows the presence of coumarins. |
| Detection of tannins |
| Gelatin Test: To the extract, 1% of gelatin solution and to this sodium chloride was added. Formation of white precipitate indicates the presence of tannins. |
| Detection of phenols |
| Ferric chloride test:Extracts were treated with 3-4 drops of ferric chloride solution. Formation of bluish black colour indicates the presence of phenols. |
| Detection of anthraquinone |
| To the extract, magnesium acetate solution was added the pink colour developed indicates the presence of anthraquinone. |
| Detection of quinone |
| Few mg of the substrate in alcohol is treated with sulphuric acid, the colour developed indicates the presence of quinone. |
| Detection of steroids and terpenoids |
| 10 mg of extract dissolved in chloroform and few drops of acetic anhydride added by 1 ml of the concentrated sulphuric acid and blue to green shows the presence of steroids and pink colour in chloroform layer shows the presence of terpenoids. |
| Detection of catechin |
| Few mg of the substrate in alcohol is treated with a few drops of Ehrlich reagent and a few drops of concentrated HCL, the pink colour developed indicates the presence of catechin. |
| Detection of saponins |
| Extract dissolved in water and shaken well, the froth which last for a long time shows the presence of saponins. |
IV. RESULTS AND DISCUSSION |
| The expansion of safe, effective and low cost anti-HIV agents are the top global priorities of drug development, since the long term complications of this disease are multifactorial and can be related to the virus itself or adverse effects of antiretroviral therapy.Extraction was performed by the microwave assisted extraction method using six solvents (ethanol, methanol, water, petroleum ether, chloroform and n-hexane) followed by one or two cycle extraction at different temperatures. |
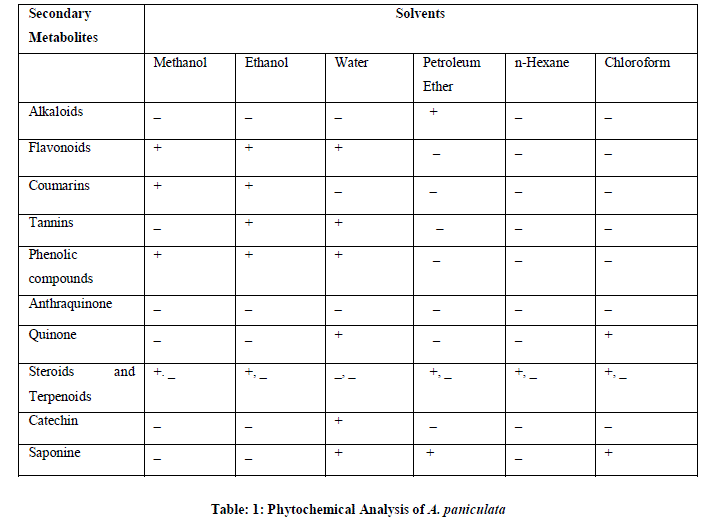
| Table 1 shows the phytochemical analysis of A.paniculata plantof the ethanol, methanol, water, petroleum ether, chloroform and n-hexane extracts. It was performed in order to know the presence of different phytocompounds such as Alkaloids, Coumarins, tannins, Phenolic compounds, Anthraquinones, Quinones, Steroids and Terpenoids, Catechin, saponines. Results obtained showed the presence of Flavonoids, Tannins, Phenolic compounds, Steroids in A.paniculata plant extracts, and less amount of Coumarins, Anthraquinone and Terpenoids were absent in these extracts. |
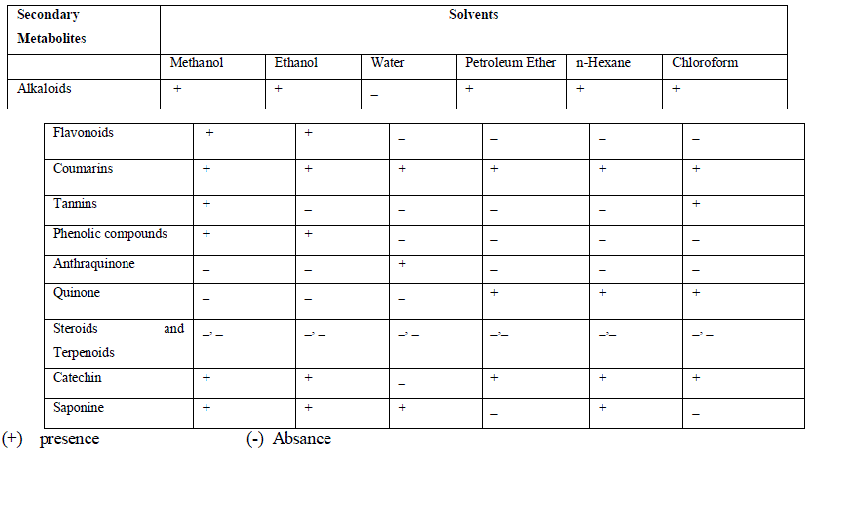
| Table 2 shows the phytochemical analysis of C.verum plant of the ethanol, methanol, water, petroleum ether, chloroform and n-hexane extracts. It was performed in order to know the presence of different phytocompounds such as Alkaloids, Coumarins, tannins, Phenolic compounds, Anthraquinones, Quinones, Steroids and Terpenoids, Catechin, saponines. Results obtained showed the presence of Alkaloids, Flavonoids, Coumarins, Catechin, Saponine in A.paniculata plant extracts, and less amount of Phenolic compounds, Anthraquinone and Tannines, Steroids and Terpenoids were absent in different solvent extracts. |
 |
 |
V. CONCLUSION |
| Plants are an important source of anti-HIV chemical compounds, and several plant families and speciescontain anti-HIV bio-active compounds that could be developed into newer drugs to manage HIV/AIDS. Thus the present study does seem to justify the raditional use of plants for the treatment of infectious disease of viral origin, However, in order to assess the usefulness of these herbs, it is necessary to isolate the active principles from crude extracts, identify them and study their mechanism of action. |
References |
|