ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
A. S. Fouda1, M.N. EL-Haddad1 and Y.M.Abdallah2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The effect of an antibacterial Septazole on the corrosion of copper was studied in 0.1 M HCl at different concentrations using potentiodynamic polarization, electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS) measurements. Potentiodynamic polarization indicated that Septazole acted as mixed- type inhibitor. EIS measurements were also used to investigate the mechanism of corrosion inhibition. Results obtained from EFM technique were in agreement with potentiodynamic and EIS techniques. The adsorption of Septazole on the surface of copper followed the Langmuir adsorption isotherm at all concentrations and temperatures studied. The reactivity of Septazole was analyzed through quantum chemical calculations based on PM3 semi-empirical method to explain the efficiency of this inhibitor as corrosion inhibitors.
Keywords |
| Septazole, copper, acid corrosion, PM3 semi-empirical calculations. |
I. INTRODUCTION |
| Copper is used in many types of industrial and electronics applications due to its easily available, economically suitable, excellent electrical, thermal conductivity and a relatively noble metal, requiring strong oxidants for its corrosion or dissolution. It is well-known that corrosion products have a negative effect on heat transfer on the copper based heat exchanger, which can be reduced by periodic cleaning in hydrochloric acid pickling solutions. Corrosion inhibitors could effectively remove some of the undesirable reactions connected with destructive effects of hydrochloric acid pickling solutions on the copper surface and prevent its dissolution [1-3]. Several organic compounds containing sulfur, nitrogen and oxygen hetero-atoms have been reported as corrosion inhibitors [4, 5]. The efficiency of an inhibitor is mainly dependent on its ability to get adsorbed on the surface of metal which consists of a replacement of water molecule at a corroding interface. The adsorption of this inhibitor is affected by the electronic structure of inhibiting molecules, steric factor, aromaticity, and electron density at donor site, presence of functional group such as –N=N, –CHO, R–OH etc., molecular weight and molecular area of the inhibitor molecule as reported [7–9]. A few researchers have been reported the use of antibacterial drugs as corrosion inhibitors because of presence of oxygen, nitrogen and sulphur in their structures as active centres, high solubility in water, high molecular size, non toxic “environmentally friendly” corrosion inhibitors, important in biological reactions and drugs that can be easily produced and purified [10-17]. |
| In present work the inhibitive behaviour of Septazole (4-amino-N-(5-methylisoxazol-3-yl) benzenesulfonamide) on copper in 0.1 M HCl solution has been studied using electrochemical techniques. Quantum chemical calculations were employed to explain the efficiency of this drug as corrosion inhibitor. |
II. EXPERMINTAL |
| A. MATERIALS AND SOLUTIONS |
| The working electrode was mechanically cut from cylindrical copper rod having composition (weight %): Ni 0.54; Fe 0.066; impurities 0.35 and Cu, balance. The surface of working electrode was abraded using different grades (320–1200 grade) of emery papers, degreased with acetone, washed with bidistilled water and dried with soft paper. The experimental measurements were carried out in 0.1 M HCl solution in the absence and presence of various concentrations of Septazole drug for all studies. Septazole drug was purchased from Alexandria Co. for Pharmaceuticals, Egypt. The chemical structure of Septazole (4-amino-N-(5-methylisoxazol-3-yl) benzenesulfonamide) is given in Fig. 1. The concentrations of inhibitor employed were varied from 100 to 900 ppm (mg L−1). For each experiment, a freshly prepared solution was used. |
 |
| B. ELECTROCHEMICAL MEASURMENTS |
| Polarization experiments were carried out in a conventional three-electrode cell with a platinum counter electrode (1 cm2) and a saturated calomel electrode (SCE) coupled to a fine Luggin capillary as the reference electrode. The working electrode was in the form of a square cut from copper embedded in epoxy resin of polytetrafluoroethylene (PTFE) so that the flat surface was the only surface in the electrode. |
| The electrochemical measurements were carried out using a Gamry instrument Potentiostat/Galvanostat/ZRA (PCI 300/4). This includes a Gamry Framework system based on the ESA400, Gamry applications that include DC105 for dc corrosion measurements, EIS300 for electrochemical impedance spectroscopy and EFM140 software for electrochemical frequency modulation measurements along with a computer for collecting data. Electrochemical data were analyzed by Echem Analyst 5.5 software. The working electrode was immersed in the test solution before starting the measurements, until a steady state was reached (30 min). For potentiodynamic polarization measurements, the potential was scanned at a scan rate of 1mVs−1. Potential changed automatically from -700 mV up to +300.mVSCE. EIS measurements were performed at opencircuit potential over a frequency range of 0.1Hz to 100 kHz. The sinusoidal potential perturbation was 5 mV in amplitude. EFM carried out using two frequencies 2.0 and 5.0 Hz. The base frequency was 1.0 Hz. We use a perturbation signal with amplitude of 10 mV for both perturbation frequencies of 2.0 and 5.0 Hz. |
| C. QUANTUM CHEMICAL STUDY |
| Quantum chemistry calculations were performed using the program package Materials studio 5.0 [18] with the semiempirical self-consistent field molecular orbital (SCF-MO) method to calculate the physical properties of molecular orbital of inhibitor. The geometry of inhibitor was optimized by the restricted Hartee–Fock (RHF) level using PM3 Parameterization. |
III. RESULTS AND DISSCUSSION |
| A. POTENTIODYNAMIC POLARIZATION |
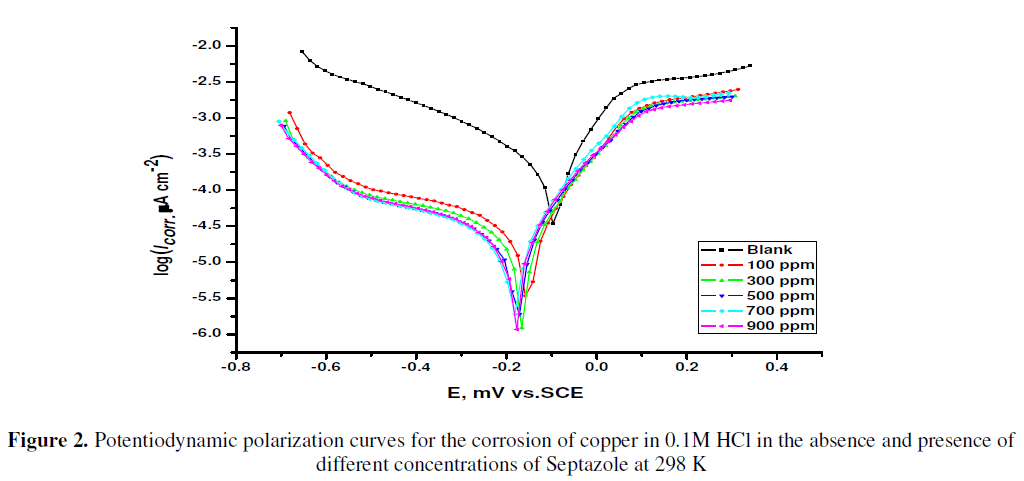
| Potentiodynamic polarization curves for the corrosion of copper in 0.1M HCl in the absence and presence of different concentrations of Septazole at 298 K are shown in Fig.2. The corrosion current density (Icorr), anodic (βa) and cathodic (βc) Tafel slopes were calculated by extrapolation of linear parts of anodic and cathodic curves to the corresponding corrosion potential (Ecorr). The percentage inhibition efficiency (IEPP %) and the degree of surface coverage (θ), were calculated from equation (1) [19]: |
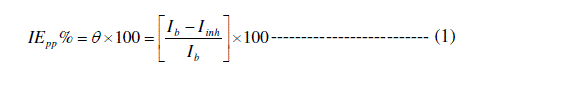 |
| Where, |
| Ib= The corrosion current density of uninhibited solution. |
| Iinh = The corrosion current density of inhibited solution. |
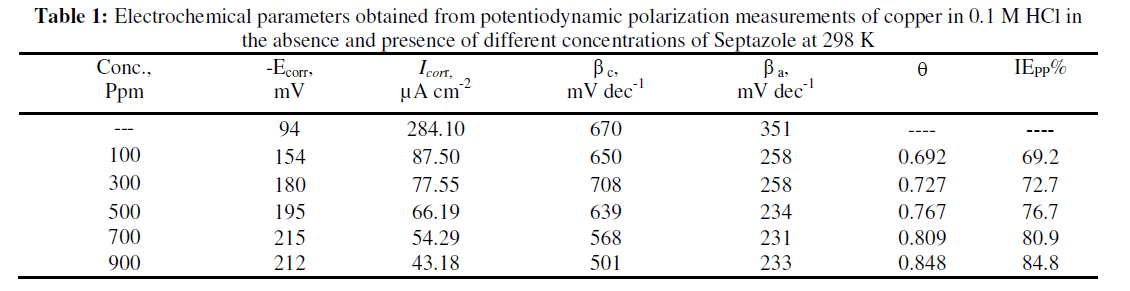
| The electrochemical parameters, determined from Tafel polarization curves are summarized in Table 1. The data in Table 1 showed that the current density decreased in the presence of Septazole than in its absence. The % inhibition efficiency increased with increasing Septazole concentration. This indicates that Septazole molecules are adsorbed on the metal surface. The inhibition was more pronounced with increasing inhibitor concentration. Tafel lines are shifted to more negative and more positive potentials with respect to the blank curve by increasing the concentration of the inhibitor (Fig. 2), also there is a small changes in Ecorr value. This behavior indicates that Septazole acts as mixed-type inhibitor [20]. |
 |
 |
| B. ELECTROCHEMICAL IMPEDANCE SPECTROSCOPY (EIS) MEASURMENTS |
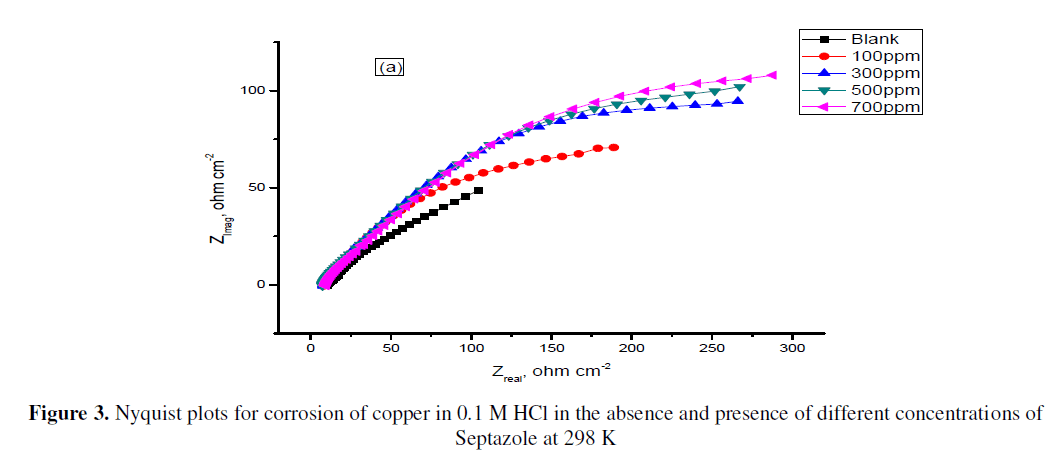
| Nyquist plots of copper in 0.1 M HCl solution in the absence and presence of different concentrations of Septazole at 298 K are shown in Fig. 3. The charge transfer resistance (Rct) values were calculated from the difference in the Nyquist plots at low and high frequencies. Therefore, the inhibition efficiency, (IEEIS %) and the degree of surface coverage (θ) of Septazole can be calculated from the charge- transfer resistance according to Eq. (2) [21]: |
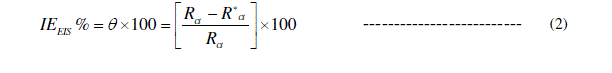 |
| Where, |
 |
| Where, |
| fmax = The frequency at the maximum in the Nyquist plot. |
| Rct = the values of charge transfer resistance. |
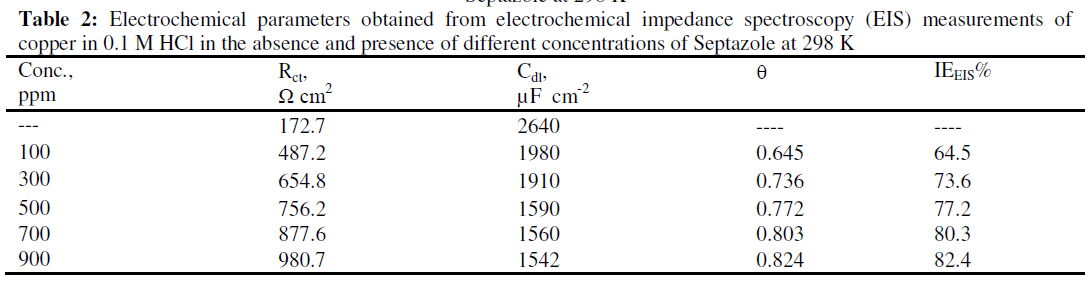
| Cdl = double layer capacitance obtained from the Nyquist plots and the calculated inhibition efficiency values (IEEIS %) are reported in Table 2. |
| It is apparent from this table that the value of Rct increased with increasing concentration of Septazole. The increase in Rct values is attributed to the formation of an insulating protective film at the metal/solution interface. So that. The (IEEIS %) increased. On the contrary, the value of Cdl decreased upon the addition of the inhibitor, suggesting, a decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer, indicating the inhibitor molecules function by the formation of the protective layer at the metal surface [23]. |
 |
 |
| C. ELECTROCHEMICAL FREQUENCY MODULATION (EFM) MEASURMENTS |
| EFM is a nondestructive and a rapid test technique. Also, The EFM technique is used to calculate the anodic and cathodic Tafel slopes as well as corrosion current densities without prior knowledge of Tafel constants. Fig. 4a and b show representative examples for EFM intermodulation spectra of copper in 0.1 M HCl in the absence and presence of 900 ppm of inhibitor at 298 K. Similar results were obtained for the other concentrations. The peaks of spectrum are analyzed by the EFM140 software to calculate the corrosion current and the Tafel constants. The inhibition efficiency (IEEFM %) and the degree of surface coverage (̩̉) can be calculated from equation (4) : |
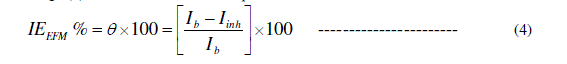 |
| The electrochemical parameters obtained from spectral analysis of EFM are summarized in Table 3. It is clear that from this table, the values of Icorr decrease with increasing the concentration of inhibitor, this means (IEEFM %) increased, suggesting that Septazole inhibits the corrosion process by adsorption on the copper surface. The values of causality factors (CF-2, CF- 3) are very close to theoretical values (2) and (3) according to the EFM theory, suggesting that, the validity of Tafel slopes and corrosion current densities [24, 25]. |
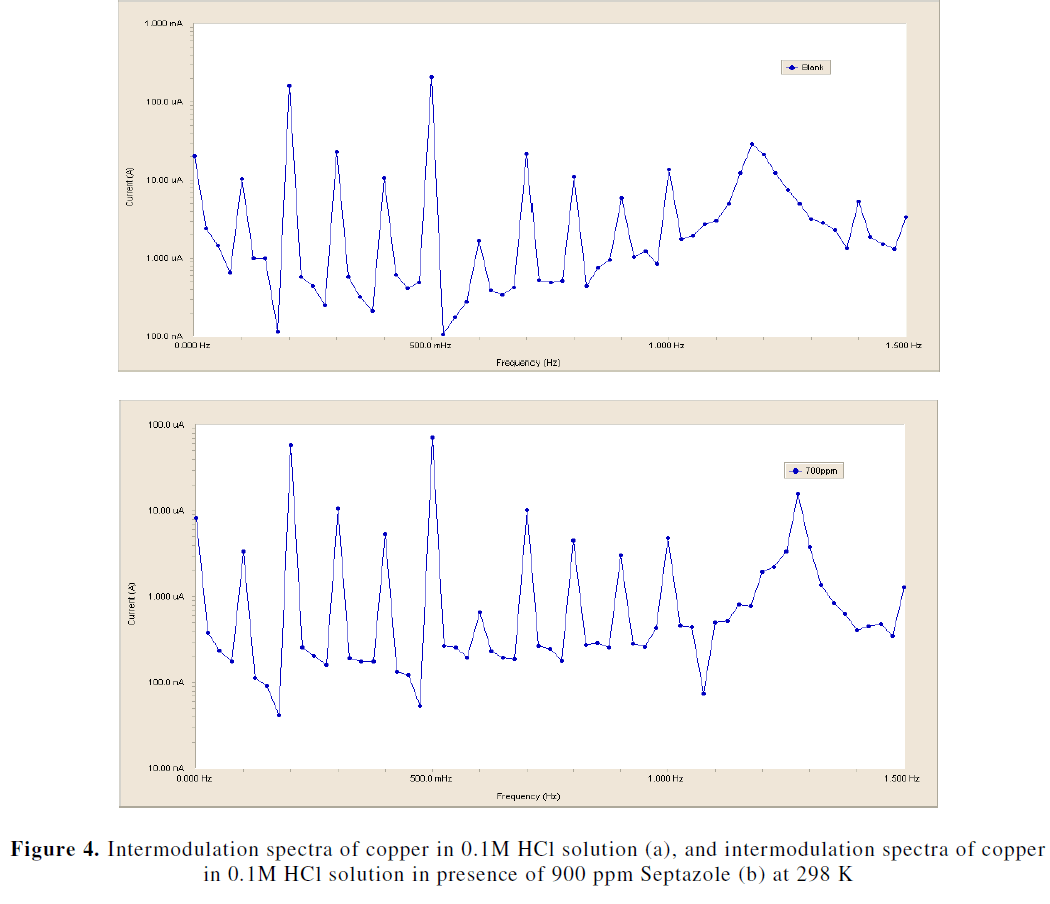 |
 |
| D. ADSORPTION ISOTHERM AND THERMODYNAMIC PARAMERTS |
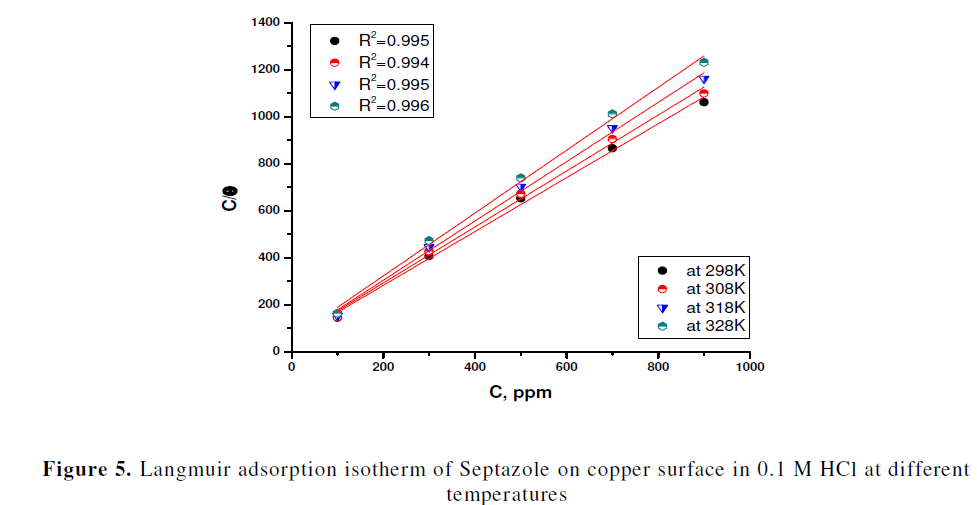
| Basic information on the interaction between the surface of copper and inhibitor can be determined from several adsorption isotherms. The common used adsorption isotherms are the Temkin, Frumkin, Langmuir and Flory–Huggins isotherm. The degree of surface coverage (θ) for different concentrations of the inhibitor (Cinh) has been evaluated. The data were tested graphically to determine a suitable adsorption isotherm. A straight line with linear correlation coefficient (R2) is almost equal to 1.0, was obtained on plotting Cinh/θ versus Cinh at all studied different temperatures as shown in Fig. 5, indicating that adsorption of the inhibitor on the copper surface obeys the Langmuir adsorption isotherm. According to the Langmuir adsorption isotherm, the surface coverage (θ) is related to inhibitor concentration (Cinh) by equation (5) [26]: |
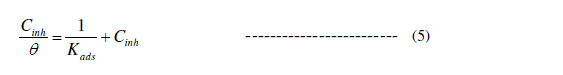 |
| Where, |
| Kads = the adsorption equilibrium constant. |
| The slopes of straight lines (Fig.5) were found to be very close to unity. This means that, adsorbed molecule occupies only one site and it does not interact with other adsorbed species [27]. The Kads values can be calculated from the intercept lines on the Cinhi/ θ axis in Fig.3. Kads is related to the standard free energy of adsorption (ïÃÂÃâG0 ads) by equation (6) [26]: |
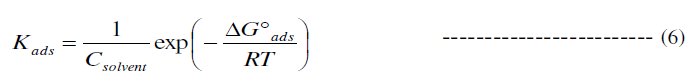 |
| Where, |
| Csolvent = molar concentration of the solvent, which in the case of water is 55.5 mol L−1. |
| R = the gas constant |
| T = the absolute temperature. |
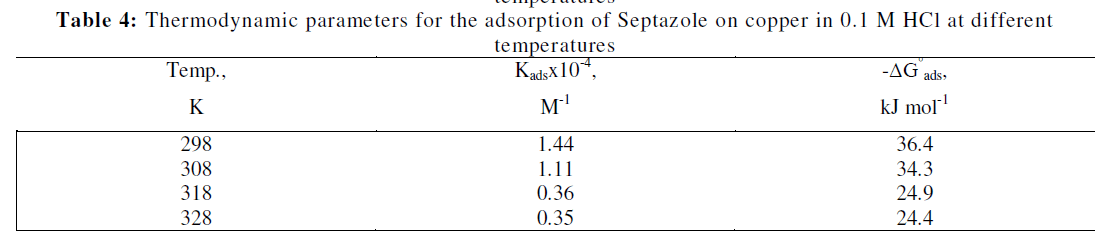
| The calculated values of Kads and ïÃÂÃâG0 ads are given in Table 4. As shown from Table 4, the large values of Kads obtained for investigated drug, imply efficient adsorption and, therefore, better inhibition efficiency. The negative value of ïÃÂÃâG0 ads, indicated spontaneous adsorption of the inhibitor on the surface of copper. Generally, the value of ïÃÂÃâG0 ads up to - 20 kJ mol-1 suggests electrostatic interactions between the charged molecules of inhibitor and the charged metal surface (i.e., physisorption). On the contrary, the value of ïÃÂÃâG0 ads above − 40 kJ mol−1, involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate bond (i.e., chemisorptions). In our measurement, the calculated value ïÃÂÃâG0 ads at 298 K for copper is - 36.4 kJ mol−1, which suggests that adsorption of the inhibitor on the copper surface involves both physical and chemical process [28, 29]. But the inhibition efficiency decreased with increasing temperature, indicating that the inhibitor adsorbed predominantly physically on the copper surface [30]. |
 |
 |
| E. EFFECT OF TEMPERATURE AND KINETIC PARAMETARS |
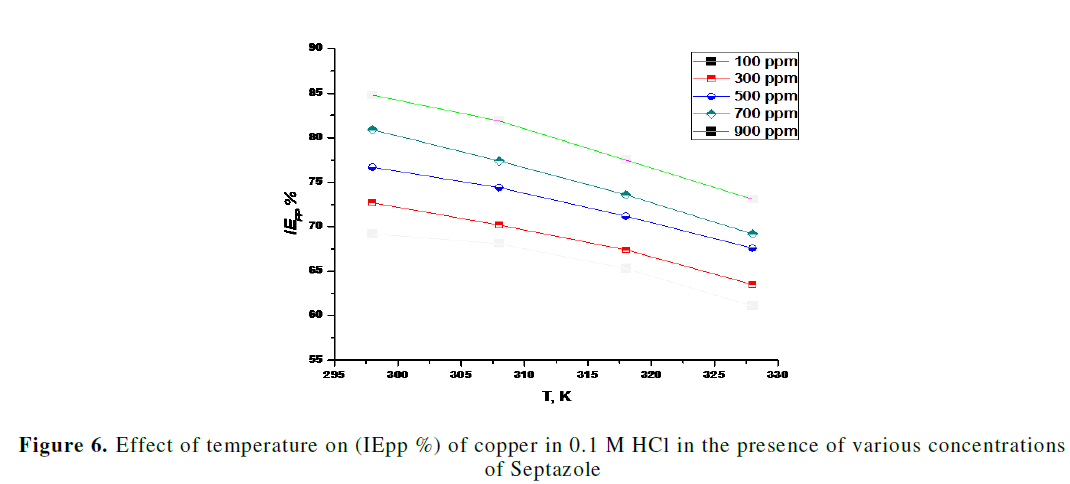
| To evaluate the mechanism of inhibition and to determine the kinetic parameters of the corrosion process, potentiodynamic polarization measurements were carried out at 298- 328 K. The effect of temperature on the corrosion inhibition efficiency (IE %) of copper in the presence of different concentrations of the inhibitor is represented in Fig. 6. As shown from Fig. 6, the inhibition efficiency (IE %) decreased with increase in temperature, and hence, protective film of inhibitor formed on the surface of copper is less stable at higher temperatures, which may be due to the desorption of some adsorbed molecules from the surface of the copper, due to which greater area of the metal is exposed to the acidic environment as reported [27]. The apparent activation energy (Ea) of copper corrosion in 0.1 M HCl can be expressed using the Arrhenius equation (7) [31]: |
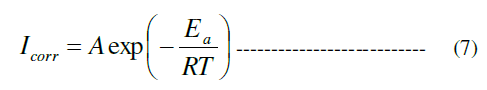 |
| Where, |
| Icorr = the current density. |
| Ea = the apparent activation energy (kJ mol-1). |
| A = the Arrhenius pre-exponential factor. |
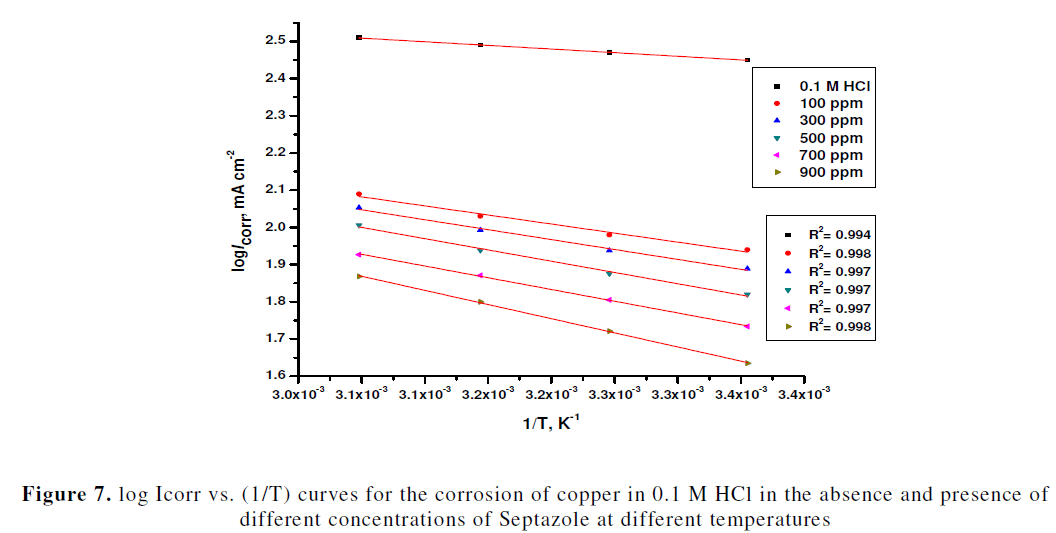
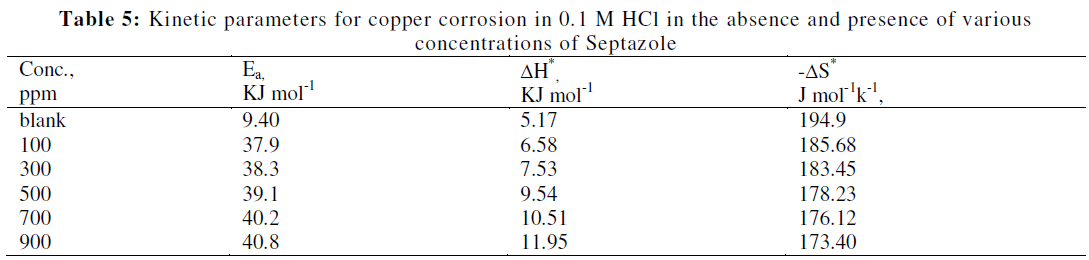
| Fig.7 shows, the Arrhenius plot of log Icorr against 1/T for the corrosion of copper in 1 M HCl solution in the absence and presence of various concentrations inhibitor. From Fig.7, the values of Ea were calculated from the slope of each individual line using the expression Ea = (slope) x 2.303R, and then, are listed in Table 5. It is clear from Table 5, that the values of Ea for the inhibited solutions were higher than that for the uninhibited solution, suggesting a physical adsorption. The increase in Ea can be attributed to an appreciable decrease in the adsorption of the inhibitor on the copper surface with increase in temperature. Similar behaviour was previously reported [32, 21]. The values of the enthalpy of activation (ïÃÂÃâH*) and entropy of activation (ïÃÂÃâS*) were calculated by equation (8) [31]: |
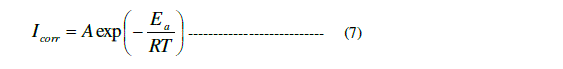 |
| Where, |
| h = Planck’s constant . |
| N =the Avogadro’s number. |
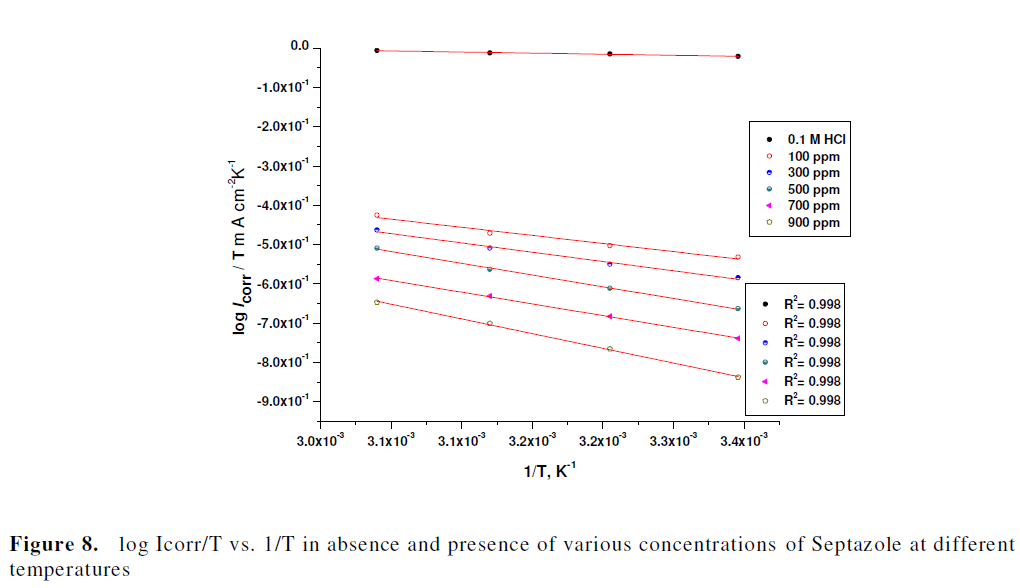
| A plot of log Icorr/T vs. 1/T. (Fig. 8), gave straight lines with slope of (-ïÃÂÃâH*/2.303R) and an intercept of, {log(R/Nh) + ïÃÂÃâS*/2.303R} from which the values of ïÃÂÃâH* and ïÃÂÃâS* were calculated and summarized in Table 5. The positive values of ïÃÂÃâH* for corrosion of copper in the presence and absence of the inhibitor reflect the endothermic nature of the metal dissolution process as reported [33]. The entropy of activation values are less negative for inhibited solutions than that for the uninhibited solutions. This indicates that an increase in randomness occurred while moving from reactants to the activated complex as reported [34, 35]. |
 |
 |
 |
 |
| F. QUANTUM CHEMICAL CALCULATIONS |
| Quantum-chemistry calculations have been performed in order to study the molecular structure and the reaction mechanisms in order to interpret the experimental results as well as to solve chemical ambiguities and to correlate the inhibition efficiency to the molecular properties of inhibitor. The optimized geometry of the inhibitor and distributions of Mulliken charge, as well as the nature of their molecular orbitals, HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) are shown in Fig.9. According to the frontier molecular orbital theory, the formation of a transition state is due to an interaction between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of reactants [36]. Quantum chemical parameters related to the molecular electronic structure such as, EH0MO, ELUMO, energy gap (ïÃÂÃâE = ELUMO - EHOMO) and the dipole moment (μ) were calculated. It is found that, a high EHOMO values ( -9.19 eV) of the molecules leads to higher electron donating ability to appropriate acceptor molecules with low energy empty molecular orbitals. A low ELUMO values (-0.94 eV) suggests that the molecule accepts electrons easily from donor molecules. The difference ïÃÂÃâE = ELUMO - EHOMO is the energy required to move an electron from HOMO to LUMO. The smaller value of ïÃÂÃâE (8.25 eV) facilitates adsorption of the molecule and thus will cause higher inhibition efficiency, because the energy to remove an electron from the last occupied orbital will be low [37]. Low value (4.44 eV) of the dipole moment (μ) will favour the accumulation of inhibitor molecules on the metallic surface. From the values of Mulliken charge (Fig. 9) we can observe the presence of excess of negative charge on nitrogen, oxygen and sulfur atoms which can be adsorbed on the copper surface using these active centers leading to the corrosion inhibition action. |
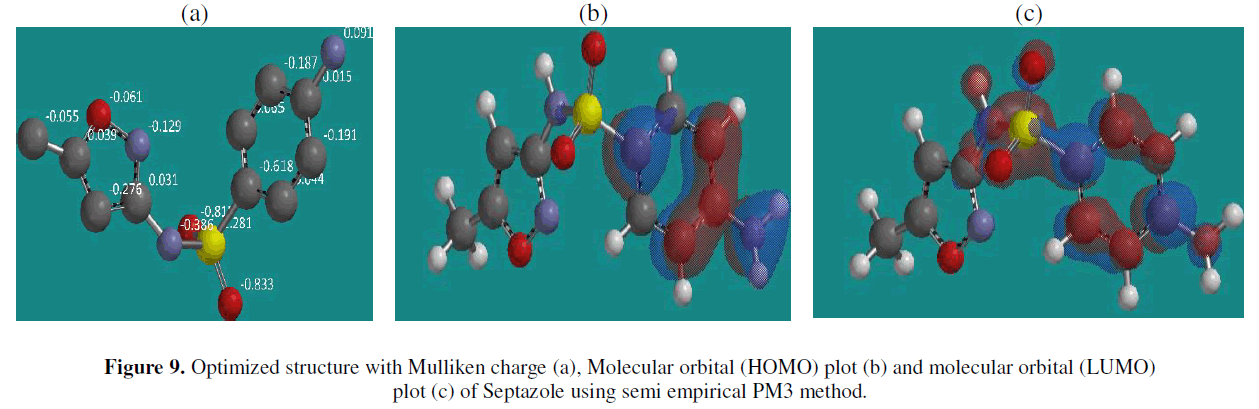 |
| G. MECHANISM OF CORROSION INHIBITION |
| The adsorption of investigated drug can be attributed to the presence of polar unit having atoms of nitrogen, sulphur and oxygen and aromatic/heterocyclic rings. Therefore, the possible reaction centers are unshared electron pair of hetero-atoms and ÃÂû-electrons of aromatic ring [38]. The adsorption and inhibition effect of Septazole drug in 0.1 M HCl solution can be explained as follows: In general, two modes of adsorption are considered on the metal surface in acid media. In the first mode, the neutral molecules may be adsorbed on the surface of copper through the chemisorption mechanism, involving the displacement of water molecules from the copper surface and the sharing electrons between the hetero- atoms and Cu. The inhibitor molecules can also adsorb on the copper surface. In the second mode, since it is well known that the copper surface bears positive charge in acid solution [38], so it is difficult for the protonated molecules to approach the positively charged copper surface due to the electrostatic repulsion. Since chloride ions have a smaller degree of hydration, thus they could bring excess negative charges in the vicinity of the interface and favor more adsorption of the positively charged Septazole molecules, the protonated Septazole adsorb through electrostatic interactions between the positively charged molecules and the negatively charged metal surface. Thus there is a synergism between adsorbed Cl- ions and protonated Septazole. Thus we can conclude that inhibition of copper corrosion in 2 M HCl is mainly due to electrostatic interaction. The decrease in inhibition efficiency with rise in temperature supports electrostatic interaction. |
IV. CONCULSIONS |
| From the overall experimental results the following conclusions can be deduced: |
| 1. The investigated drug is good inhibitor and act as mixed type but mainly act as anodic inhibitors for copper corrosion in 1 M HCl solution. |
| 2. The results obtained from all electrochemical measurements showed that the inhibiting action increases with the inhibitor concentration and decreases with the increasing in temperature. |
| 3. Double layer capacitances decrease with respect to blank solution when the plant extract is added. This fact confirms the adsorption of plant extract molecules on the carbon steel surface. |
| 4. The adsorption of inhibitor on copper surface in HCl solution follows Langmuir isotherm for septazole. |
| 5. The values of inhibition efficiencies obtained from the different independent quantitative techniques used show the validity of the results. |
| 6. Quantum chemical parameters for septazole were calculated to provide further insight into the mechanism of inhibition of the corrosion process. |
References |
|