ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Mashal M.H. Al-Sobay1 and Samy M. El-megharbel1,2 Moamen S. Refat1,3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
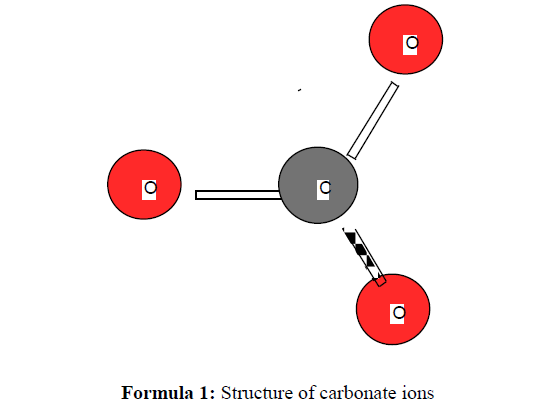
The work reported in this study deals with synthesis, characterization and structural investigation of some new compounds including novel methods for the synthesis of some metal carbonates. Metal(II) carbonate, MCO3, is obtained by a new very simple synthetic method involving the reaction of aqueous solutions of some metal ions with urea at ~ 90 oC. The infrared spectrum of the formed product clearly indicates the absence of bands due to urea and shows the characteristic bands of carbonate ion. The (CO3 2-) ion is planar and therefore, it belongs to the D3h symmetry. It is expected to display four modes of vibration, A` 1 + A" 2 + 2E` (E` is a doubly degenerate motion). The zinc(II) and cobalt(II) carbonates, respectively, were performed using a new chemical procedure through the interaction of Zn(HCOO)2.xH2O and Co(HCOO)2.xH2O in aqueous solution with a urea as a simple organic precursor at ~ 90 oC for 12 hrs.
Keywords |
| carbonate CO3 2–, infrared spectra, cobalt(II) nitrate, zinc(II) nitrate, urea. |
I.INTRODUCTION |
| Urea is physiologically very important. It is the chief nitrogenous product of protein metabolism. Urea has a melting point of 132 ïÃâðC, soluble in water and ethanol, but insoluble in ether. Urea is used for preparing formaldehyde-Urea resin (plastics) [1], barbiturates [2], and fertilizers [3-6]. Urea is also extensively used in the paper industry to soften cellulose and has been used to promote healing in infected wounds and many other applications in the field of medicine [7-9]. Some metal-urea complexes have pharmaceutical application, e.g., the platinum-urea complex which is used as antitumor [10]. Yamaguchi and Stewart [11, 12] were assigned all of the observed frequencies in the spectra of urea and urea-d4. The two vibrations of the frequencies at 1686 and 1603cm-1 were assigned as the 1686 cm-1 band due to CO stretching vibration and the 1603 cm-1 band for NH2 bending motion. The calculations studied by Yamaguchi showed that for the band at 1686 cm-1, the contribution of the NH2 bending motion is greater than that of CO stretching motion. The infrared bands of urea-d4 observed at 1245 and 1154 cm-1 are assigned to ND2 bending vibrations. This assignment is consistent with the observed depolarization degrees of the Raman lines. The 1464cm-1 frequency of urea is assigned to the CN stretching vibration. The corresponding frequency of urea-d4 is observed at 1490cm-1. The 1150cm-1 band is assigned to NH2 rocking vibrations. The reactions between transition metal ions and urea at room temperature have been studied extensively [13-17]. The infrared spectra of these complexes clearly indicated that urea molecule behaves as a mono dentate ligand and coordinates to the metal ions through the oxygen atom and not the nitrogen atom. The nature of the reaction products depend strongly on the type of metal ions and so the metal salt used. The novelty in our previously studies [18-27] were oriented to the reaction of urea ligand with different metals such as Co(II), pb(II), Sn(II), Cr(III), Fe(III), Au(III), Sn(IV), V(V) and Mo(IV) at high temperature which demonstrate that the types of metal ions beside their anions have a pronounced effect on the nature of the reaction products. The published papers were trended for the reaction of urea with different metal salts at elevated temperature lead to discovering a novel method for preparation pbCO3 and CoCO3 [21], lanthanide carbonates [23, 27], limonite, FeO(OH) [20], 2ZnCO3.3Zn(OH)2 [19], SnOCl2.2H2O [18], (Cr2O3, MnO2, MoO3 and WO3) oxides resulted from a novel oxidation reduction reaction between (K2CrO4 or K2Cr2O7), KMnO4, Na2MoO4 and Na2WO4, respectively, with urea in an aqueous solution at ~ 85 oC [27]. The shining point in this paper was aimed to identify the reaction mechanisms of the products resulted during the reaction of urea with Zn(HCOO)2.xH2O and Co(HCOO)2.xH2O at 90 oC for 12 hrs in aqueous media. The reaction products were isolated as solids and characterized by infrared spectroscopy |
II.EXPERIMENTAL |
| 2-1- Materials and synthesis |
| All chemicals used throughout this paper were analytical pure. MCO3 (M = Co(II) and Zn(II)) were prepared by mixing an aqueous solutions (50 ml) of 0.1M of urea with 0.01M of the respective Co(HCOO)2.xH2O and Zn(HCOO)2.xH2O, respectively. The mixtures were heated at 90 oC for 12 hrs in a hot plate. The solid products compounds were filtered off, washed several times with hot water, dried at 90 oC in an oven. The yields of the obtained Co(II) and Zn(II) carbonates were varied in the range 55-to-60% depending upon the type of metal as well as on the counter ions associated with the metal ion. |
| 2-1- Instruments |
| Carbonate content in the cobalt(II) and zinc(II) compounds were determined by dissolving a sample of each product in excess standard HCl and the excess of HCl was determined using standard sodium carbonate [28]. The percentage of cobalt(II) and zinc(II) in their compounds were determined gravimetrically method till constant weight and stable oxides formula. The infrared spectra of urea, all reactants and products were recorded using a Bruker FT-IR Spectrophotometer in Taif University spectroscopic Laboratory. |
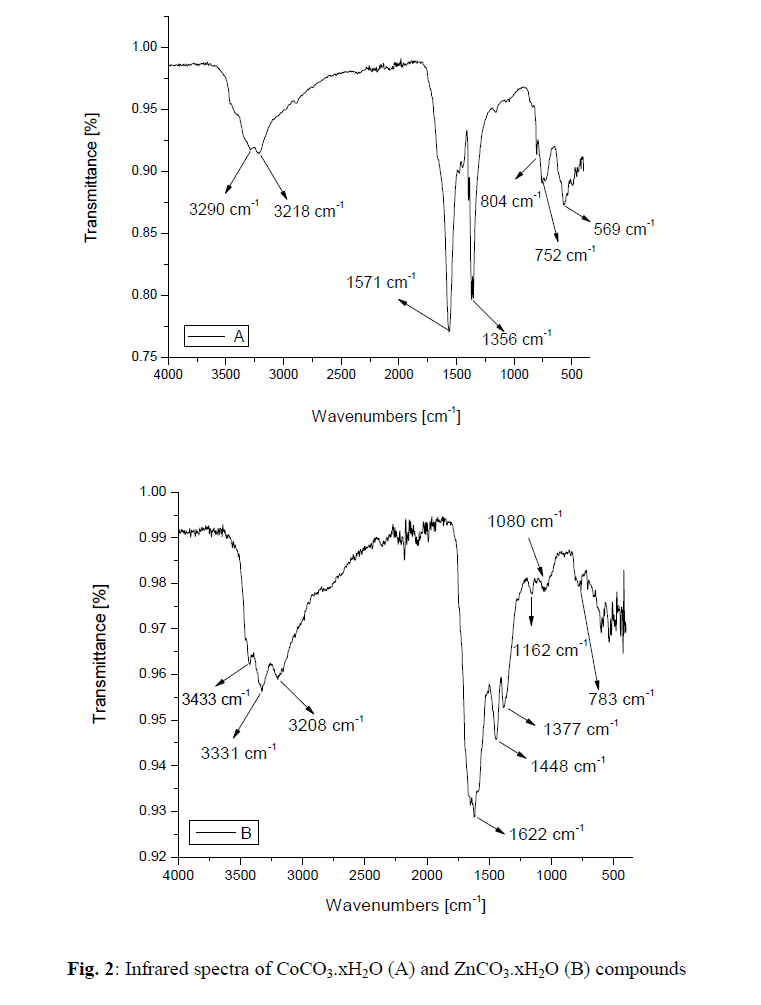
III.RESULTS AND DISCUSSION |
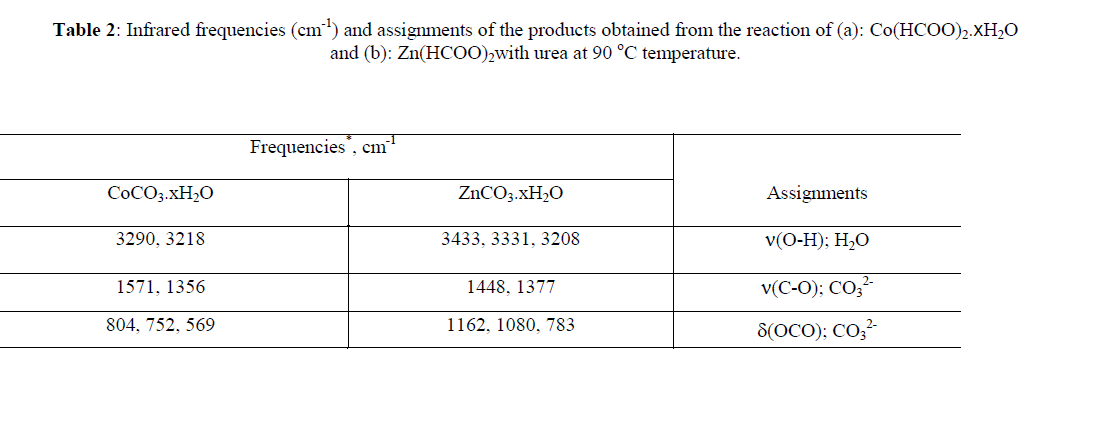
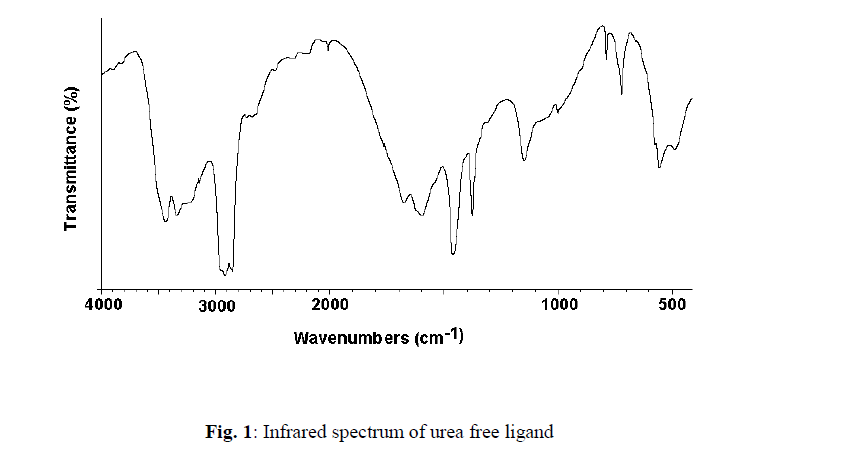
| The reaction of aqueous solutions of urea with formate salts of cobalt(II) and zinc(II) at 90 o C produces a pink and white solid powder products, respectively. The infrared spectra of urea as well as the reaction products of different cobalt(II) and zinc(II) salts with urea at high temperature less than boiling points of water solvent were obtained. The spectra of free urea ligand, cobalt(II) and zinc(II) carbonates are shown in Fig. 1 and 2, respectively. The band assignments for the products are given in Table 1. The infrared spectra show no bands due to any of the reactants and of coordinated urea, but instead, a group of bands characteristic for the ionic Carbonate, (CO3)2-, is appeared [29]. Based on this fact, the volumetric determination of (CO3)2- group with standard solution of HCl and beside that the infrared spectra of the commercially obtained CoCO3 and ZnCO3 are the same as that of the reaction products. The products obtained were identified as CoCO3.xH2O and ZnCO3.xH2O. The infrared assignments agree quite well with those known [29] for the ionic carbonate (CO3)2-. Previous studies [18-27] indicated that the nature of the reaction product obtained from the reaction of metal ions with urea at high temperature depends upon the type of metal ion and in some cases on the nature of the metal salts used. Oxalato urea complexes of manganese(II)[MnU2(C2O4)]H2O (U=Urea) was prepared [30] from the reaction of MnC2O4.2H2O and urea at pH ~3 in 1:1, 1:2, 1:3 and 1:4 molar ratios. This complex was characterized by its IR-spectrum, X-ray diffraction, and thermal analysis. The data obtained indicates that, the oxalato group and the H2O molecule are in the inner-sphere. The thermal analysis data indicate that, this complex [MnU2(C2O4)]H2O decomposes at 400ïÃâðC in three stages to Mn3O4 as the final product. Taking the [MnU2(C2O4)]H2O parent complex as aguid in our paper, so, at high temperature the role of Co(II) ions in decomposing the coordinated urea in the form of [CoU2(HCO2)2] could be understood according to the following reactions; |
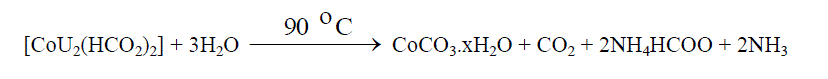 |
 |
 |
 |
 |
References |
|