ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Anil Kumar K1, Dr Srinivasu Ch2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Sound velocity, densities of binary mixture of 1,4-dioxane with 1-hexanol has been measured over the entire range of composition using Anton Paar at (T=298.15, 303.15, 308.15, 313.15 & 318.15) K. The Excess parameters viz., deviations in isentropic compressibility (ΔKs), excess molar volumes (Vm E), excess free length (Lf E), excess acoustic impedance (ZE) , excess sound velocity (uE) are deduced from experimental values and discussed intermolecular interactions present in the mixture. At the end, all the parameters have been fitted to Redlich-Kister equation and their coefficients are obtained.
Keywords |
| Sound velocity; Densities; Excess parameters; 1,4-dioxane ; 1-hexanol |
I. INTRODUCTION |
| Day to day knowledge of thermodynamic and transport properties liquid/liquid mixtures are essential for the proper design of any chemical industrial processes. 1,4-dioxane is used in polymerization processes is a cyclic ether, an excellent solvent very often used in the manufacture of special chemicals, pesticides, bulk drug intermediates and 1-hexanol is a substance heavily used in the pharmaceutical industry as a solvent, an extraction agent, in processes that produce medicine flavors and in wastewater treatment [1,2]. According to Lagemann and Dunbar et al [3] pointed out the sound velocity approach for the qualitative estimation of interaction in liquids. A parallel measurement of sound velocity, density of liquid mixtures allows one to obtain information about their excess compressibility, volume, free length, acoustic impedance, internal pressure and changes in their properties. Complex formations, formation of hydrogen bond, Dipole – dipole, dipole- induced dipole interactions in solutions and their effect in physical properties of the mixture have received much attention. Also, Romero et al [4] measured speeds of sound of the binary mixtures of 1,3-dioxolane (or 1,4-dioxane) + cyclopentane (or cyclohexane, or benzene) at 283.15, 298.15, and 313.15 K. The excess isentropic compressibilities were calculated from experimental data and fitted with a Redlich-Kister polynomial function. Results were analyzed taking into account molecular interactions and structural effects in the mixtures. |
| In this paper, sound velocity, density of binary mixture 1,4-dioxane with hexnaol have been measured at (T=298.15, 303.15, 308.15, 313.15 & 318.15) K over the entire range of composition using Anton Paar DSA 5000M. From the experimental values, deviations in isentropic compressibility (ΔKs), excess molar volumes (Vm E), excess free length (Lf E), excess acoustic impedance (ZE), and excess sound velocities (uE) for the binary system are estimated using standard equations that are reported by several authors [5-9]. The results obtained were fitted to Redlich–Kister polynomial the results were discussed in terms of molecular interactions. |
II. EXPERIMENTAL |
| Materials & Measurements: The measurements were performed using the sound analyzer incorporated in the Anton Paar device, model DSA 5000M, equipped with the machine sampler SP-1m (Anton Paar; carousel with 24 vials, 55 cm3 each). All controls, adjustments, and checks were done using manufacturerâÃâ¬ÃŸs software system put in within the device. A laptop connected to the U tube densimeter enabled us to read the raw data from the device memory and to perform the ensuing analysis. Mole fractions of these samples were determined by measuring the mass of each component with a precision balance (Sartorius, model CP 225D, +/-0.01mg). |
| 1,4-dioxane and 1-hexanol with mass fraction purities >0.998 were purchased from Aldrich Chemical Co. the chemicals were kept in airtight stopped glass bottles were performed in an isothermal mode; that is, the measurements of all prepared solution were done at the similar explicit temperature, then the temperature was changed and the measurements were perennial. The instrument in calibrated with the double distilled water (as suggested by Anton-Paar) for sound velocity and density at room temperature. The accuracy of density measurements was 5*10−6 g·cm−3, and also the temperature of the equipment was maintained constant to within ± 0.01 K. |
 |
III. THEORY |
| Using the measured values of data, we can calculate the various thermo acoustical parameters such as Isentropic |
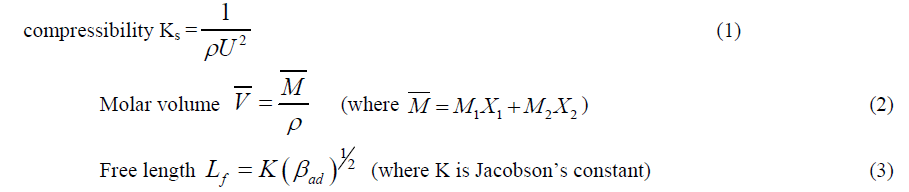 |
| Specific acoustic impedance Z = U ρ (4) |
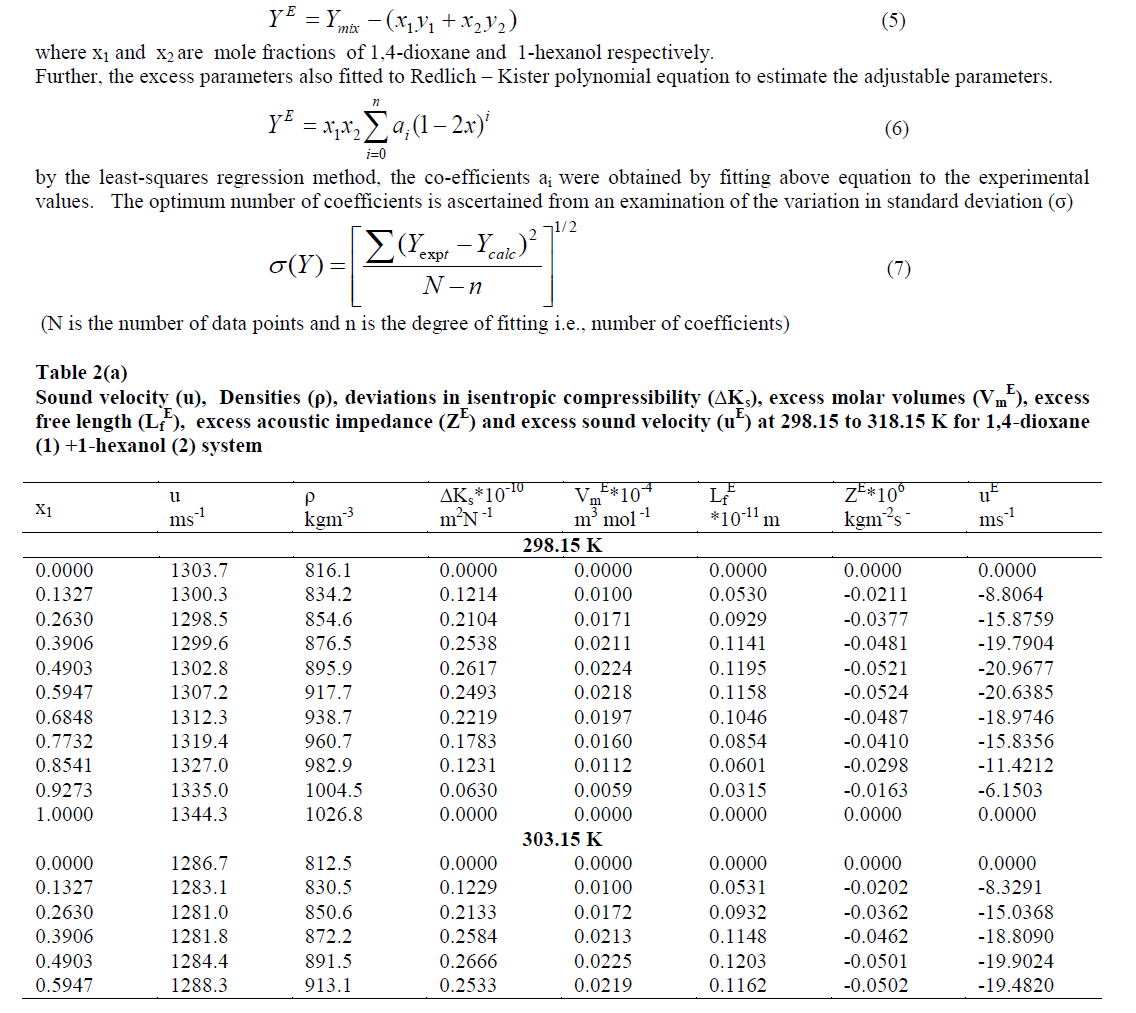
| The strength of interaction between the component molecules of binary mixtures is well reflected in the deviation of the excess functions from ideality. The excess thermodynamic properties such as ΔKs, Vm E, Lf E, ZE and uE have been calculated using the following equation |
 |
 |
 |
 |
 |
IV. RESULTS AND DISCUSSION |
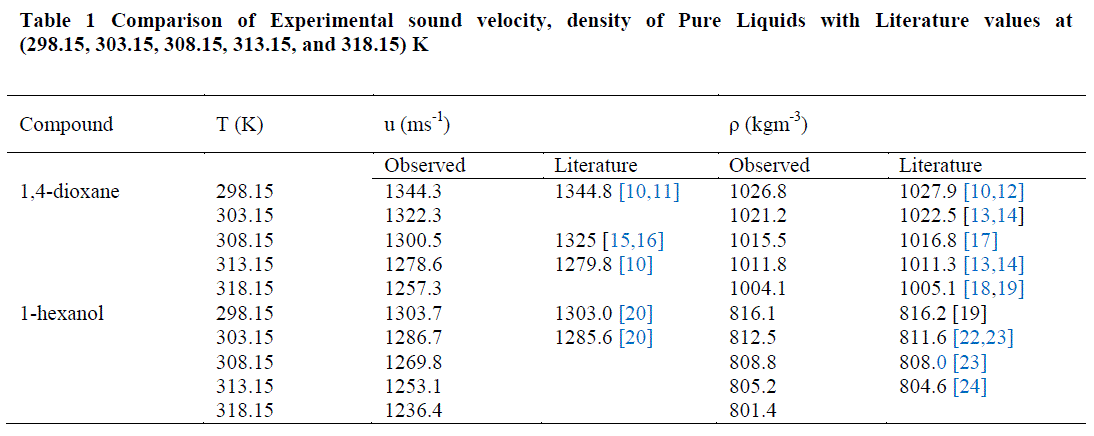
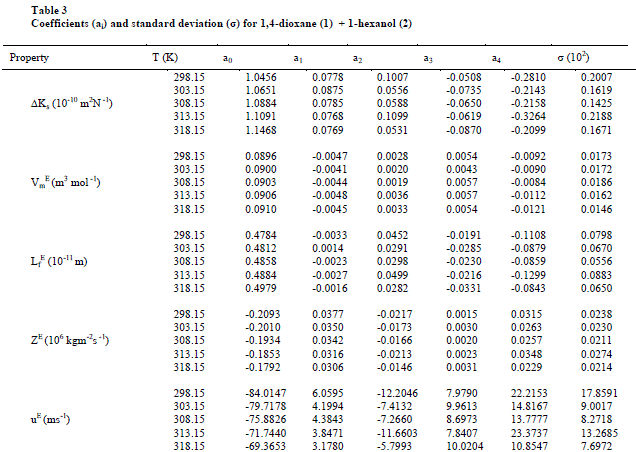
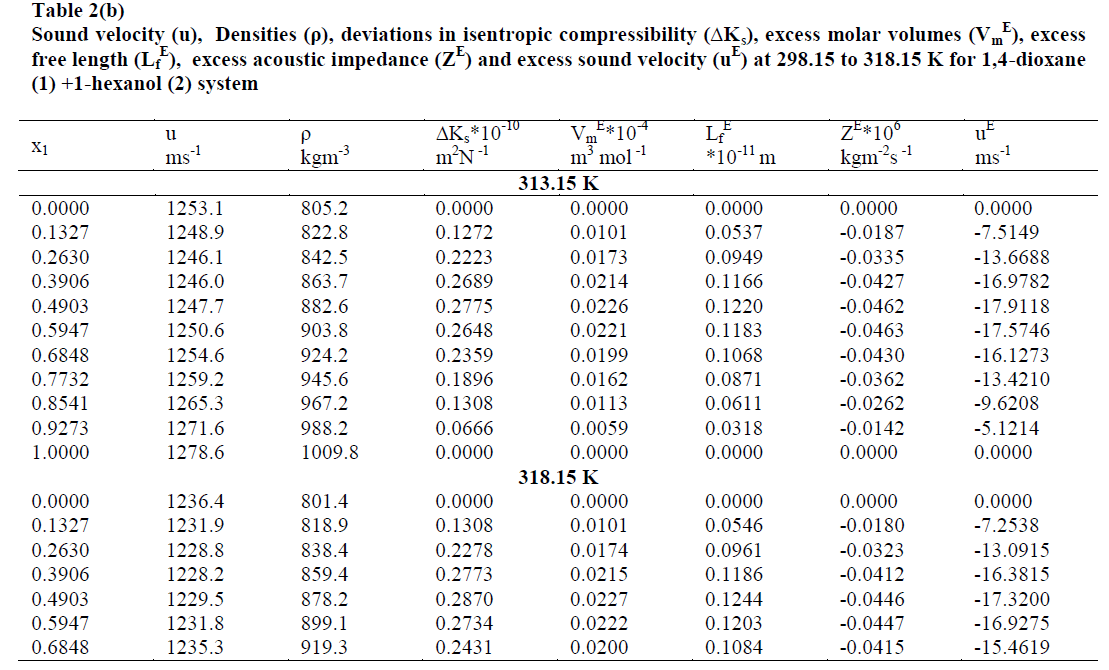
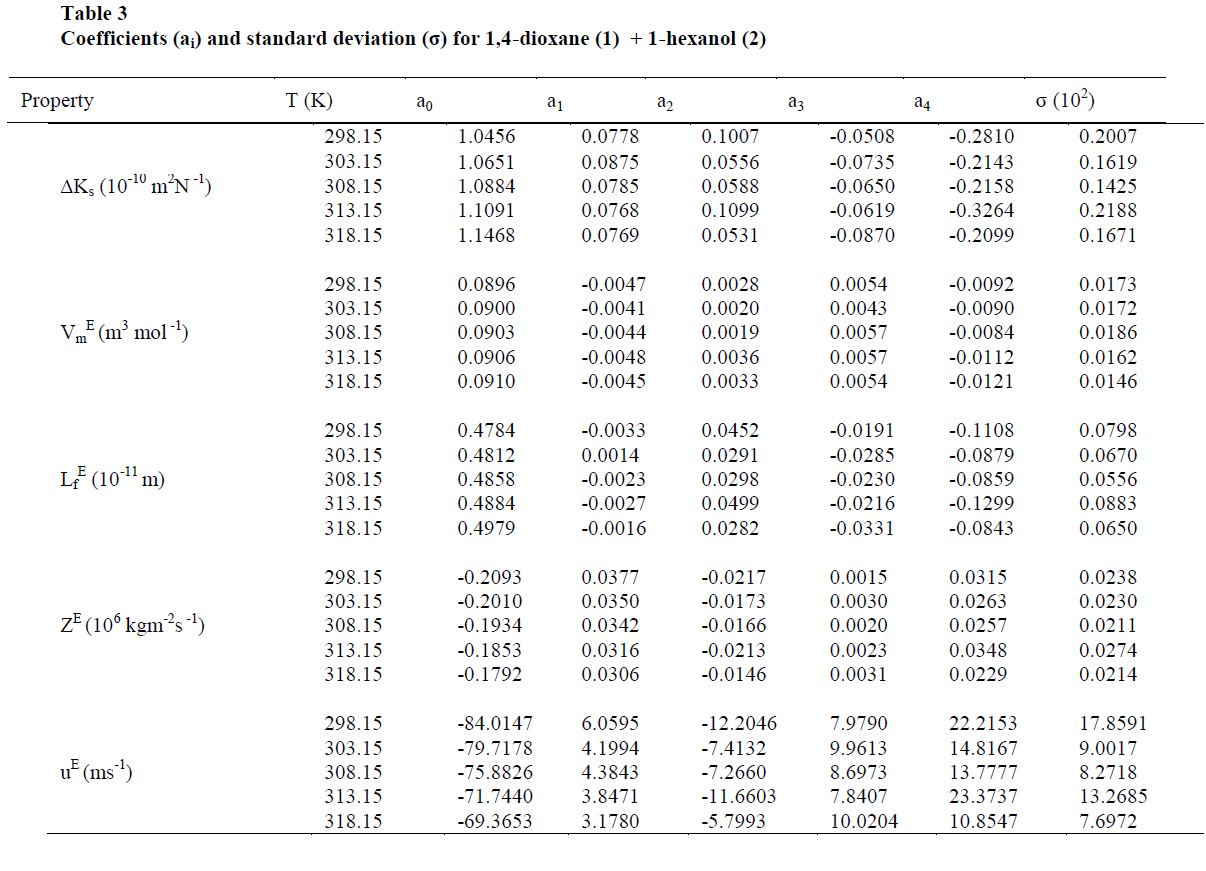
| The values of sound velocities and densities for pure liquids are experimentally measured and are compared with the literature values and they are good agreement with each other as given in the table-1. The experimental data related to ΔKs, Vm E, Lf E, ZE and uE for the binary liquid mixture at different temperatures are given in tables-2 (a), 2 (b). Redlich-Kister coefficients of the various parameters at different temperatures with respective standard deviations are reported in table- 3. |
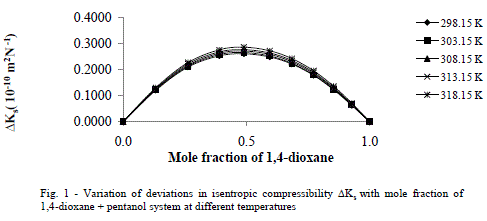
| Deviation in Isentropic Compressibility (ΔKs) |
| The values of deviation in isentropic compressibility for 1, 4-dioxane + 1-hexanol binary mixture studied at five different temperatures are given in tables 2 (a), 2 (b).The corresponding plots of deviation in isentropic compressibility against mole fraction of 1, 4-dioxane are plotted in Fig 1. The ΔKs plots are more positive at intermediate composition x1 =0.5. The positive values of ΔKs suggest that the mixture is more compressible than the corresponding ideal mixture. This leads decrease of strength of the interactions between component molecules in the mixture. |
 |
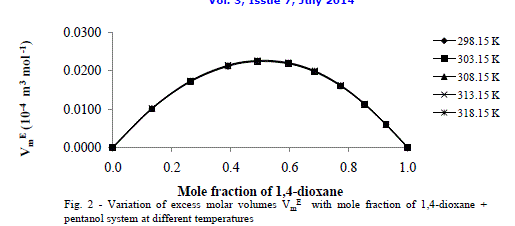
| Excess molar volume (Vm E) |
| Fig.2 shows the excess molar volumes Vm E in the case of 1, 4-dioxane +1-hexanol are found to be more positive at x1 = 0.5 at all temperatures studied. The positive values of excess molar volumes suggest that the mixture prefers to have loose structure than compact structure in the solutions [25] i.e. weak intermolecular interactions are present in the mixtures. |
 |
| Excess free length (Lf E) |
| The values of „Lf EâÃâ¬ÃŸ for 1,4-dioxane +1-hexanol binary mixture studied at five different temperatures are presented in tables 2(a), 2(b) respectively, and there corresponding plots against mole fraction of 1,4-dioxane are shown in Fig. 3 shows more positive values at x1=0.5over the entire range of composition of 1,4-dioxane at all five temperatures studied. According to Wankhede [26] the positive values of Lf E is an indication of dispersive forces are operating in the liquid mixtures and it is showing the presence of weak interactions in the mixture .This also reveals the presence of week interactions in these mixtures. |
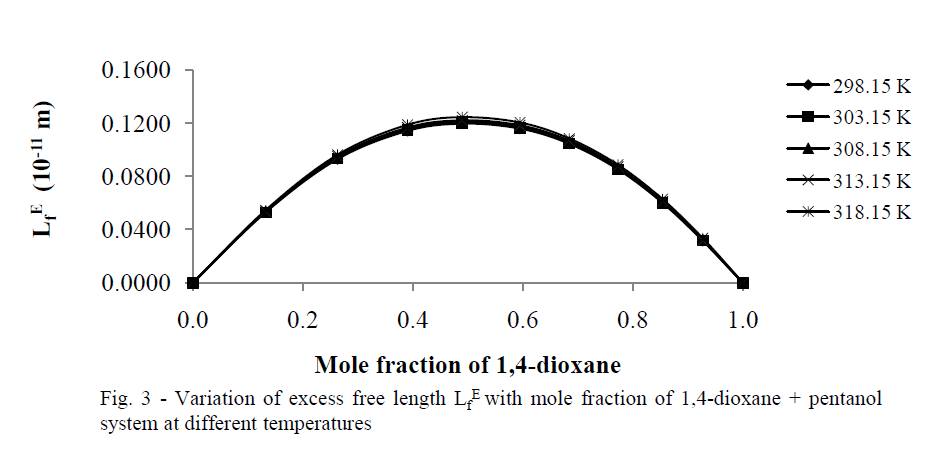 |
| Excess Acoustic Impedance (ZE) |
| Further, the calculated values of excess acoustic impedance (ZE) of binary mixture at all temperatures studied are negative. The corresponding plots are shown in fig- 4 shows; the excess acoustic impedance becomes more negative at 0.5947 mole fraction of the binary mixture. The negative values ZE [6,27] suggests that breaking of hydrogen bond in 1-hexanol up to 0.5947 and after that it decreases and hence it leads to close packing of the structure. This shows weak molecular interactions between the components of the mixture exists. |
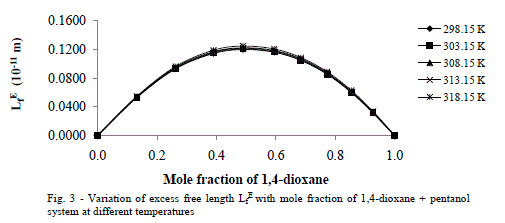 |
| Excess Ultrasonic Velocity (uE) |
| Figs 4,5 show that behaviors of ZE and uE support each other both exhibit negative deviations over the entire composition range of 1,4-dioxane in the mixture at all five temperatures. The negative deviations suggested dispersion forces are operative in the system. Similar observations are reported by A Ali et al [8] on the binary mixtures 2,2,4-trimethyl pentane with n-hexane and cyclohexane. |
 |
| On close observation of Table 2(a), 2(b) the values of ΔKs and Vm E are positive at all temperatures studied. An increase in ΔKs denotes highly compressible, weakening of inter-molecular interactions i.e., the hetro molecular (1,4-dioxane + 1-hexanol) not only disturb the homo molecular (1,4-dioxane molecules), (1-hexanol molecules) interactions in components liquids but also causes a re-arrangement in the geometry of the clusters of molecules in such a way volume of the mixture i.e., excess molar volume would be positive [28]. The sign and magnitude of ΔKs and Vm E play a vital role in assessing the molecular interactions in the liquid mixtures. In general negative values of ΔKs and Vm E indicates strong interaction in the mixture which include charge-transfer, diploe-dipole, dipole-induced dipole interactions and interstitial accommodation of the smaller molecules into the spaces created by bigger molecules, while positive signs of these parameters are indicative of weakening of interactions between the component molecules [29]. |
| Also, the values of ΔKs & Lf E (Fig 1,3) are found to be positive over the entire range of mole fraction of 1,4-dioxane in the mixture at all studied temperatures. The sign of excess properties plays a vital role in assessing the compactness or extent of molecular interactions. The various types of interactions that are operating between the molecules are dispersion forces, which should make a positive contribution to excess values and charge transfer, H-bonding, dipole-dipole interaction and dipole-induced dipole interactions expected to make positive contributions. In the present mixture as ΔKs & Lf E are positive suggesting dispersive forces are present in the mixture [26]. |
V. CONCLUSION |
| Sound velocity and density for binary mixture consist of 1,4-dioxane with 1-hexanol system are measured at T=(298.15 to 318.15) K using Anton-Paar. The calculated excess parameters are discussed and concluded the presence of weak dispersion forces in the mixture. |
VI. ACKNOWLEDGEMENT |
| The author (AK) is grateful to the Andhra Loyola College (Autonomous) NAAC re-accredited at „AâÃâ¬ÃŸ grade with 3.65/4.00 points, Vijayawada-8. For allowing the author to avail laboratory facilities of the Department of Physics, thank s extended to Dr Ch Srinivasu for giving fruitful discussions and suggestions. |
References |
|