E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Yulian Zhao1, Qunwei Dai1,2*, Faqin Dong1,2, Linbao Han1, and Wang Yan1
1School of Environment and Resource, Southwest University of Science and Technology, Mianyang 621010, PR China
2Key Laboratory of Solid Waste Treatment and Resource Recycle, Ministry of Education, Mianyang, Sichuan Province, 621010, PR China
Received date: 02/02/2016; Accepted date: 26/05/2016; Published date: 06/06/2016
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
The present study was conducted to determine the abilities of Pseudomonas fluorescens cells, montmorinment and their composites to accumulate strontium from a liquid medium, and elucidate the role of microbes on the mobility of strontium. Firstly, strontium (under 1000 ppm) did not have effect on the metabolism of P.fluorescens, according to the results of growth curve and FTIR. Various P. fluorescensmontmorinment system were exposed to solution of 100 ppm Sr2+ in a liquid medium to differentiate its removal efficiency. Experimental results showed that the removal rate of strontium reached 93.62%, 93.38%, respectively, in the montmorillonite-P.fluorescens contact/ non-contact system. And the removing process could be divided into slowly removal phase (0-80 h) and rapidly removal phase (80-120 h). The relative strontium removal capacities by actual soil, in decreasing order, were actual soil>P.fluorescens>sterilized soil - P.fluorescens system>sterilized soil. Results of strontium removal by the components of system showed that EPS and dead cells played the major role in removing strontium in the montmorillonite - P.fluorescens system. These results suggest that P. fluorescens play an important role in removing strontium by the montmorillonite - P.fluorescens system.
Montmorinment, Pseudomonas fluorescens, Strontium, Removal, Mineral - microbe system
With the urbanization, human population and drawing of natural resources expanding, which are closely associated with intensification of industrial activities such as mining, mill tailing, metal finishing, electroplating, nuclear power testing, nuclear waste disposal, electricity generation in nuclear reactors and accidents resulting from nuclear power generation, contribute to the environmental contamination by hazardous metals and radionuclides [1-4]. Nuclear power poses potential threats to living organisms and the environment due to the long-lasting radioactive pollutants produced from nuclear leakage and unsuitable disposal of nuclear waste [5-6]. Radioactive pollution is gaining increasing attention with the recent bloom of nuclear plants. Among the radioactive pollutants, 90Sr is one of the most harmful radioactive nuclides, and it comprises one of the greatest proportions of radionuclides released from the Fukushima Daiichi Nuclear Power Plant accident in 2011 due to the magnitude 9.0 earthquakes and tsunami. There are several methods to manage the radionuclide pollution, such as physical method, chemical method and phytoremediation method [7-9]. However, there are some deficiencies in these methods. There has been a tremendous amount of attention given to the use of mineral - microorganism systems for removal of radionuclides and heavy metals from solutions.
Bentonites have been proposed as buffer materials in the geological disposal system of high level nuclear waste [10]. With montmorillonite as a principal component, bentonites are expected to strongly adsorb fission products such as 137Cs and 90Sr. Montmorillonite is a constituent clay mineral of various soils, sediments in reducing environments, and the major component of bentonite, which is a candidate material for the backfill of radioactive waste repositories [11]. Montmorillonite acts as a sorbent for a variety of metal cations, including Pb (II) and U (VI). Montmorillonite clays are minerals with great specific surface and optimal properties for metal adsorption. They are also able to complex kinds of organic and polymeric compounds on its surface [12]. The clay minerals montmorillonite have been tested extensively as adsorbents in the removal of toxic heavy metals from aqueous solutions a few years ago. The works which focused on how the clays and their modified forms (following acid activation, intercalation and pillaring) have been used as adsorbents to remove heavy metals from water and how the experimental conditions like pH, temperature, adsorbent amount and adsorbate concentration influenced the adsorption capacities have been investigated.
Biological methods are inexpensive and effective for treating metal wastewaters and in situ bioremediation of metal (loid) contamination, little progress has been made towards metal (loid) recovery [13]. Microbes such as bacteria, fungi and algae are widely used as metal adsorbents. And its quantity can be as high as 1012/g soil [14]. Bacteria are ubiquitous in natural soil. Bacteria are able to sequester trace elements in their cell and to release them again [15]. As we known, microorganisms are often intimately intermixed with mineral phases to form complex, highly hydrated, high surface-area composite materials through attractive van der Waals interactions, hydrogen bonding, or ion bridging.
Montmorillonite clays and bacteria have noticeable metal sorption capacity. Clays or bacteria are difficult to separate from the solution when used as sorbent materials [16]. Graeme F. Morley showed that mixtures of montmorillonite and fungal biomass have reduced uptake of metals [17]. Nagina Parmar showed that non-viable S.alga cells and cell envelopes can sorb significantly greater quantities of Sr2+ [18]. Chen studied the abilities of the living and nonliving P. putida CZ1 cells, clays and their composites to accumulate Copper and Zinc from a liquid medium. Their results showed that bacteria play an important role in regulating the mobility of heavy metals in the soil environment [19]. Although most studies have examined metal binding by using isolated, singlecomponent systems, such as purified clays or bacterial walls and provide information on basic mechanisms of metal sorption, it does not apply directly to metal behavior in soils, which are significantly more complex. Much work is devoted to heavy metal sorption and relevant mechanisms by biology and minerals [20-24], but fewer studies focused on the removal of strontium by the interaction between bacteria and mineral through contact/non-contact system, which was the representative acted place of strontium in soil.
The aim of this present study was to evaluate the strontium removal capacity of montmorinment - P. fluorescens system, including montmorinment - P. fluorescens contact and non-contact system, living cells, dead cells and EPS.
Preparation of microorganism
Pseudomonas fluorescens (Gram-negative), isolated from a lawn soil in the Sichuan Basin, China, was identified by its morphology and 16S rRNA gene sequence (GenBank accession number: HM468063). This strain is a normal soil inhabitant, especially found in meadow soil. The size of bacterial cell is 0.7-0.8 μm × 2.3-2.8 μm. Before inoculation, the culture was maintained on agar slants and stored at 4°C. The sugary culture medium was composed of (per L) 6 g of glucose (GLU), 3 g of beef extract, 10 g of peptone, and 5 g of NaCl. The pH of the culture solution was adjusted to 7.0-7.2 with hydrochloric acid or sodium hydroxide. Under the above conditions, P. fluorescens was incubated at 35°C during 24 h stationary culture.
Preparation of clay
Bentonite was collected from the Altay area of Xinjiang Province in China to study the influence of the normal soil bacteria on interlayer characteristics of clay mineral. The chemical composition of bentonite as determined from XRF was, in percent by mass: SiO2, 59.65; Al2O3, 18.06; Fe2O3, 4.22; MgO, 2.54; CaO, 1.41; Na2O, 2.92; K2O, 1.77; MnO, 0.03; TiO2, 0.39; P2O5, 0.04; SrO, 0.04; loss on ignition, 8.90. The bentonite used in this study was ground and sieved to collect grains < 200 mesh (approximately 75 μm).
Preparation of strontium solution
Strontium solutions (0, 10, 25, 50, 100, 250, 500, 1000, 5000 ppm) were prepared by diluting 10000 ppm strontium stock solution which was obtained by dissolving a weighed quantity of strontium in nitrate salt form, Sr(NO3)2, analytical grade. Diluted solutions were prepared at room temperature in ultrapure water (15.0 MΩ cm). Then the concentration of the strontium solution is diluting to 0, 10, 25, 50, 100, 250, 500, 1000, 5000 ppm, respectively.
Experiment of bacteria growth
The experiment was conducted in 50 mL Erlenmeyer flasks containing 20 mL of strontium sugary culture medium. Strontium concentration include 0, 10, 25, 50, 100, 250, 500, 1000, 5000 ppm. P. fluorescens in their stationary phase were injected into the flasks. The injected percentage was 1:99 ( v : v ). Then blending by shakers for 1 min. 0.2 mL blending solution was injected into the honeycomb plate of Bioscreen C. The test conditional of temperature and time were 35°C, 45 h. All samples were analyzed in triplicate. The experimental treatments with strontium (100, 1000 ppm) were cultured for 1 d, 5d to analyze the changes of its surface groups, respectively.
Batch experiments of removing strontium
The experiments were conducted in 250 mL Erlenmeyer flasks containing 100 mL of strontium sugary culture medium. Strontium concentration was 100 ppm. P. fluorescens in their stationary phase were injected into the flasks. The clay stock and actual soil stock solution were prepared in the sugary cultural medium at 1.6 g/L, and then P. fluorescens was inoculated (1:99, v : v). Non-contact system was prepared by dialysis bag ( MW: 8000-14000, diameter: 36 mm ). It was used to separate the microbial cells from the montmorillonite surface. Then it was incubated on an incubator shaker (35°C, 150 rpm/min). A sample of the suspension liquid (5 mL) was then taken and centrifuged (10000 rpm, 10 min, 4°C) to obtain concentrated samples for Autoscan Advantage ICP-AES.
Analysis techniques
The growth curves of the strains were monitored using Bioscreen C (Finland). The changes of surface groups were analyzed with a Fourier transform infrared (FTIR) spectrometer (American). The concentration of residual strontium in the biosorption medium was determined using Autoscan Advantage ICP-AES (America).
Data analysis
Concentrations of Sr2+ after the removal treatment were determined by Autoscan Advantage ICP-AES. Metal removal rate (n) was determined as follows:
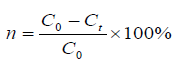
where n (%) is the removal efficiency of the Sr2+, C0 and Ct (ppm) are the initial concentration of metal ions and metal ions concentrations at the testing time.
All samples were analyzed in triplicate. Relative standard deviations (RSDs) of less than 5% were obtained for replicate samples.
Sr2+ tolerance of P.fluorescens
Growth of P.fluorescens: The growth curve of P.fluorescens with different concentrations of Sr2+ was shown in Figure 1. The results showed that the P.fluorescens could grow normally, when the concentrations of Sr2+ was under 1000 ppm. Adaptation phase continued for 7.0 h. At the time of 7.0 h, P.fluorescens began to enter the log phase. Stationary phase started from 15.0 h. When Sr2+ was added into the culture medium, P.fluorescens could grow better than control group, which was not affected by Sr2+. P.fluorescens could not grow when the concentrations of Sr2+ reached 5000 ppm. Sr2+, which concentration was under 1000 ppm, could not influence the metabolism of the P.fluorescens. As can be seen from Figure 1, the growth of P.fluorescens was best with 100 ppm Sr2+ in liquid.
FTIR analysis of P.fluorescens
FTIR result of P.fluorescens with different concentrations of Sr2+ was shown in Figure 2. When the concentrations of Sr2+ were 100, 1000 ppm, the growth time was 24 h, FTIR results of P.fluorescens was the same with the control group which was not added Sr2+. However, when the time of culture reached 120 h, FTIR results of P.fluorescens also has no changes. The FTIR analysis results showed that P.fluorescens did not exhibit any differences in their functional groups and peak positions. The results echoes with the results of growth curve (Figure 1). Therefore, when the concentrations of Sr2+ were 100, 1000 ppm, it has no effect on the metabolism of the P.fluorescens.
Analysis of Sr2+ removal by Mont-P.fluorescens contact system
The Sr2+ removal rate of montmorillonite - P.fluorescens contact system was shown in Figure 3. The Sr2+ removal rate of montmorillonite was 27.15%. At the time of 4 h and 12 h, Sr2+ removal rate of montmorillonite reached 6.89%, 8.67%, respectively, which were higher than P.fluorescens at the same time. Within 80 h, Sr2+ removal rate of P.fluorescens was very poor, only reached 24.61%. At the time of 4 h and 12 h, Sr2+ retention rate of P.fluorescens only up to 0.67%, 3.57%. The reason was that P.fluorescens was in the adaptive phase. When the time reached 80 h, Sr2+ removal rate of P.fluorescens increased rapidly. At the time of 120 h, its Sr2+ removal rate reached 96.49%. In the montmorillonite - P.fluorescens contact system, within 80 h, the maximum of Sr2+ removal rate was only 26.89%. Sr2+ removal rate of system also increased quickly when the time reached 80 h, similar to the control group of P.fluorescens. Sr2+ removal rate reached 93.62% at the time of 120 h. The initial stages of Sr2+ removal rate can be categorized as slowly removal phase (0-80 h) and rapidly removal phase (80-120 h). Montmorillonite plays major role in the initial stage. And P.fluorescens plays major role in the late phase. These data indicated montmorillonite - P.fluorescens contact system has a great potentiality for strontium retention and migrated in soil environment. The major role of mineral and bacteria was provided at different phase.
Analysis of Sr2+ removal by Mont-P.fluorescens non-contact system
Figure 4 shows the strontium removal rate by montmorillonite-P.fluorescens non-contact system. It is similar with the montmorillonite-P.fluorescens contact system (Figure 3). It is still divided into slowly removing phase and rapidly removing phase. Montmorillonite plays the major role in the initial phase, while P.fluorescens plays the key role in the later period. On the whole, bacteria play the key role in the mineral - bacteria system. The strontium removal rate of the montmorillonite was only 6.04%. While the montmorillonite-P.fluorescens non-contact system reached 93.38% at the time of 5 d. The strontium removal rate of the non-contact system was only 8.73% at 4th day.
Analysis of Sr2+ removal by actual soil
The strontium removal rate by actual soil was shown in Figure 5. For the actual soil sample, the removal capacity of strontium was highest. Sr2+ removal rate of actual soil reached 94.53%. While strontium removal rate of sterilized soil was only 10.80%. The reason may be that microbe was killed by the high-temperature (121°C) and microbe quantities were limited in the sterilized soil. Sr2+ removal rate of minerals in sterilized soil was lower than microbe, which was consistent with the effect of montmorillonite in this study. In the sterilized soil - P.fluorescens system, Sr2+ removal rate reached 88.37%. Sr2+ removal rate of P.fluorescens was up to 93.66%. Therefore, the relative Sr2+ removal capacities of this experiment, in decreasing order, were actual soil > P.fluorescens > sterilized soil - P.fluorescens system > sterilized soil. The results showed that Sr2+ removal rate increased rapidly at the third day. It could be divided into two removal phase, slowly removal phase and rapidly removal phase. For example, Sr2+ removal rate of sterilized soil - P.fluorescens system was only 19.08% at the third day. Sr2+ removal rate of actual soil was only 4.61% at the fourth day. Therefore, all these results showed that bacteria played a key role in removal strontium in the mineral-bacteria system. No matter the original bacteria of soil or the inoculation isolation bacteria, they all could improve the strontium removal capacity of the soil.
Analysis of Sr2+ removal by the components of system
Figure 6 represents strontium removal rate using Mont, live cells, EPS, and dead cells. The components of montmorillonite-P.fluorescens system include montmorillonite, live cells, dead cells and Extracellular metabolism (EPS). The results showed the relative strontium removal rate of each component used in this study, in decreasing order, were EPS>dead cells>montmorillonite>living cells. The removal rate of montmorillonite, living cells, dead cells, EPS was 27.15%, 9.12%, 58.96%, and 87.56%, respectively. It is obvious indicated that the EPS and dead cells play the major role in removing strontium in the montmorillonite-P.fluorescens system. Combined with the analysis of Figures 3-5, EPS played the dominant role in removing strontium in the montmorillonite-P. fluorescens contact/non-contact system. The possible mechanism might be that strontium can bind with the EPS because EPS has different heavy metal active functional binding sites. In the slowly removal phase, montmorillonite played key role because it’s the rapid adsorption capacity. While the concentration of montmotillonite was 1.6 g/L which was low, it reached adsorption equilibrium at early time. Therefore, before the time of 80 h, Sr2+ removal rate was lower than 30% in montmorillonite-P.fluorescens contact/non-contact system. In the rapidly removal phase, EPS played primary function might be the complexation and highly functional binding sites. After the time of 80 h, P.fluorescens was in the mature period of stability, the content of EPS reached maximum. The amount of active functional binding sites in the EPS has a great effect on removing strontium.
There are some limitations in this research. We investigated strontium removal capability in liquid by montmorillonite-P.fluorescens contact/ non-contact system. We also assessed strontium removal capability by actual soil at the same experimental conditions. We clarified that strontium removal by the components of system was in decreasing order and EPS played a major role in this system. However, this paper did not clarify the mechanisms of strontium removal by montmorillonite-P.fluorescens system, and did not indicate the main components and surface functional groups of P.fluorescens which could have highly binding capacities with strontium. Therefore, this research needed to be further studied. The paper was based on the characteristics of migrate slowly and high hazard of heavy metal in the soil, it would provide a new sight for the in-situ remediation of heavy metals and basic data.
The interaction between montmorillonite and bacteria are ubiquitous in environments, because all of them are widespread in soils. They may have profound impacts on the environment process of transporting of heavy metals or radionuclide ions. The present study concludes the effectiveness of using montmorillonite-bacteria system to remove strontium from the liquid culture. Strontium (under 1000 ppm) did not have effect on the metabolism of the P.fluorescens, according to the results of the growth curve and the FTIR. The removal rate of strontium reached 93.62%, 93.38%, respectively, in the montmorillonite P.fluorescens contact/non-contact system. And the removing process could be divided into slowly removing phase (0-80 h) and rapidly removing phase (80-120 h). The relative strontium removal capacities of the strontium removal by actual soil, in decreasing order, were actual soil > P.fluorescens > sterilized soil – P.fluorescens system > sterilized soil. The results of strontium removal by the components of system showed that EPS and dead cells play the major role in removing strontium in the montmorillonite-P. fluorescens system. These results suggest that bacteria play an important role in regulating the mobility of heavy metals in the soil environment.
This work is supported by the National Natural Science Foundation of China (No. 41130746 and 41102212) and the postgraduate innovation fund project by Southwest University of Science and Technology (No. 14ycxjj0033). Also we are grateful for the help of Analytical and Testing Center of Southwest University of Science and Technology.