ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Meenakshi Choudhary1*, Sangeeta Prasher2, Mukesh kumar3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
FTIR spectroscopy has been found to be useful technique in the study of chemical and structural changes occurring in polymeric systems due to irradiation. The present studies reports the structural changes in CR-39 polycarbonate irradiated to gamma radiations at different doses varying from 1KGy to 125KGy. The intensity of many functional groups has been found to decrease with increased dose indicating a release of some gases due to irradiation except at 5KGy dose. The G-values corresponding to some specific species have been calculated to study the reaction mechanism of polymer modificationsand the water content has been increased.
Keywords |
| CR-39, Gamma radiation, FTIR, G-value. |
I. INTRODUCTION |
| CR-39 is a polymer used in optical glasses and is found to shield UV radiations of sun. The polymer has been utilized in many dosimetry tasks. Many authors have investigated the influence of gamma radiations on the polymer so that variations in its optical properties [1] may be harnessed for gamma dosimetry. The other authors are engaged in studying the reaction mechanisms of gamma radiation induced modifications in the polymer [2]. Infrared spectroscopy can result in a positive identification (qualitative analysis) of the species released and produced during or after the irradiation process. In addition, the intensity of the peaks corresponding to the species evolved or released in the spectrum is a direct indication of the amount released and the G-value is a quantitative measure of the species produced per 100eV of the energy deposited in the conformation of the polymer. The technique has found its utilization in understand the variations in the molecular bond orders with irradiation. |
II. LITERATURE REVIEW |
| Experimental studies in various areas of material sciences and applications of these materials to other allied sciences inevitably depend on the material characteristics. The worldwide interest in the radiation chemistry of polymers arose after 1952-53, when Charlesby [3] demonstrated that polyethylene could be converted into a crosslinked, insoluble and non-melting material. |
| The effect of swift heavy ion irradiation on the radiochemistry and melting characteristics of PET was studied [4]. Radiochemistry and melting behaviour of semi crystalline polyethylene terephthalate (PET) had been studied by FTIR. The absorbance bands at around 1044, 1095, 1370 and 1454 cm-1 were found to increase with the ion fluence. The chemical modification in PET film induced by 35 MeV/u Ar ions at fluences ranging from 1 × 1010 to 5 × 1012 ions/cm2 were studied [5]. Samples were characterized by Differential Scanning Calorimetry (DSC), Fourier Transform Infrared (FTIR) and X-ray Diffractometer (XRD). FTIR showed the increase of intensities of absorbed dose. |
| Virk and Srivastava, [6] studied the modification of optical, chemical, and structural response of CR-39 polymer irradiated by 50MeV Li3+ ion at fluence 1011 – 1013 ions/cm². The sample was characterized by UV-VIS, FTIR and XRD spectroscopy. The authors observed negligible change in FTIR spectroscopy of the sample, which implied that CR-39 had chemical stability up to fluence level of 1013 ions/cm². |
| Yamauchi et al. [7] carried out the Raman and near-IR spectral studies on the proton irradiated CR-39 plastics with 3.4 MeV at fluencies ranging from 1010 to 1014 ions cm−2. The density of –CH2– group began to decrease after the air leak subsequent to the irradiation. This implies an important role of the oxygen on forming the permanent damage along the latent track. Production of OH group and some types of radicals was confirmed by near-IR observations in the air. |
III. EXPERIMENTAL |
| For the present work, pieces of CR-39 of size 2 cm2(procured from Pershore Mouldings Ltd.) have been cut from a sheet of thickness 230 μm. The samples were washed in NaOH and then with running tap water to remove the dust impurities of the polymer. |
| For gamma irradiation the samples, were irradiated at IUAC, New Delhi using60Co as gamma source in the high vacuum radiation chamber (106 mm dia x 140 mm ht in the form of cylindrical chamber with dose rate 9 kGy/hr or 160 Gy/min) and source strength 185 TBq 5000 Ci) (fig. 2.13). A sample has been kept virgin as reference to compare the extent of modifications induced and the other samples have been exposed to the source for different times to achieve the required doses of 1, 5, 25 and 125 kGy respectively. |
| Vibrational spectroscopy has been analyzed through IR spectrum of the polymer using the latest techniques of Fourier Transform Infrared Spectrophotometer (FTIR-Perkin-Elmer) |
IV. STRUCTURAL ANALYSIS |
| The infrared spectra have been obtained for a pristine CR-39 polymer (fig.1) and for each of the exposed polymers of same thickness (fig. 2) in the transmission mode without using the normalization technique. The major infrared absorption frequencies are indicated in the infrared spectrum of pristine CR-39 polymer (fig. 1). |
| It shows the characteristic peaks at 3546, 2922, 2372 and 1759 cm-1 as belonging to H2O free stretching vibration, C-H stretching, O=C=O asymmetric stretching, C=O stretching vibration of carbonate group respectively. It has been clearly reflected from literature that the C-H stretching modes of vibration are most strong, involve carbon and hydrogen atom, and appeared at higher wavelength regime [8]. |
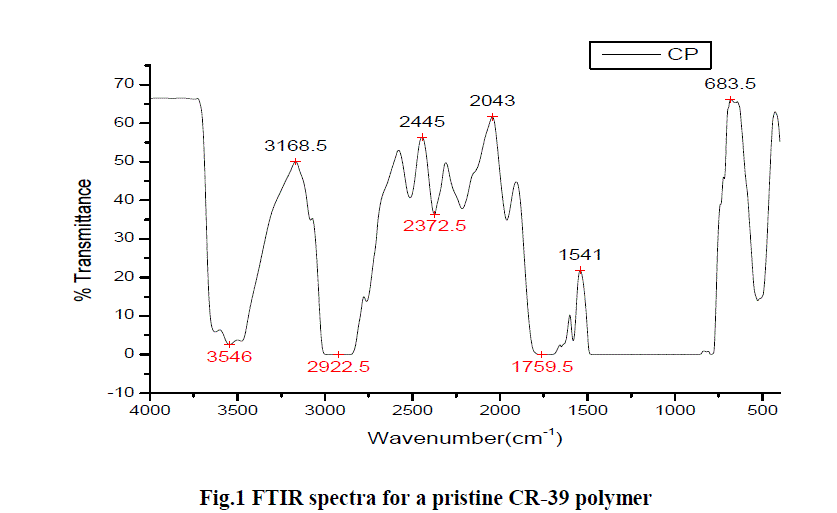 |
| There is an overall decrease in the intensity of the peaks at higher gamma doses which is given in fig. 2.Singh et al. [9] and Sun et al. [10] irradiated Makrofol detector with proton and Xe ions, respectively and reported that at higher doses peak intensity decreases. The intensity of the peak band corresponding to C=O stretch at 2372 and 1759cm-1 decreases slightly on irradiation. This may be due to chain-scission at the carbonate site [11] with the probable elimination of carbon dioxide or carbon monoxide. The intensity of the peak at 3546 cm-1 also shows a decrease in the absorbance on irradiation. Contrary to the bands above mentioned, the band at 2922 cm-1 shows increasing absorbance with the increasing dose which may show an increase in the end group of the macromolecule. |
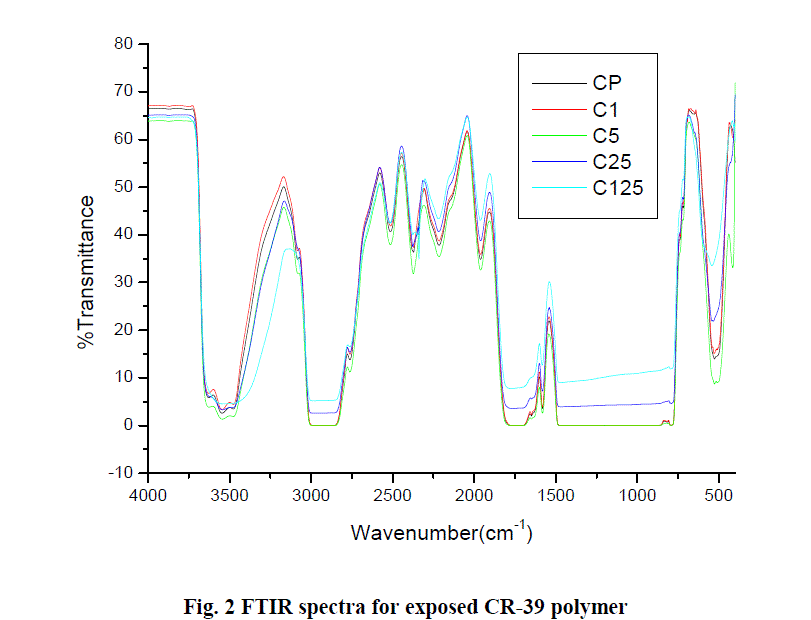 |
G value |
| The G-value is defined as the number of a particular species produced per 100 eV of energy deposited by the ionizing radiation. It can be calculated by using molar extinction coefficient as given below |
 |
| To confirm the structural changes, studied by transmittance spectrum of gamma irradiated CR-39;G-value has been calculated and reported in table 1. |
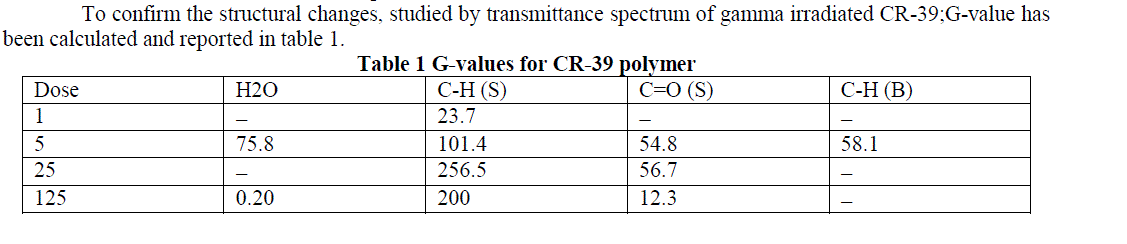 |
| The G-value is calculated corresponding to different transmittance bands.The intensity of the peak for CR-39 at 3546 cm-1 show a decrease in the absorbance on irradiation and the corresponding G-value also shows a decrease in free H2O content from 75.8 to 0.20. A decrease in the G-value corresponds to bonding of polymeric species, which imply that the water content of the sample has been increased. The peak intensity of C=O stretching also shows a decrease, which is further confirmed by the corresponding decrease in G-value from 54.8 to 12.3 at higher doses. The G-value for C-H stretching has been found to be increased which signify the breaking of bonds; similar trend has been shown by transmittances spectrum of C-H stretching, where the intensity of the peak shows an increase. |
V. CONCLUSION |
| FTIR spectroscopy has been found to be useful technique in the study of chemical and structural changes occurring in polymeric systems due to irradiation. Overall decrease in intensity of all the functional groups has been noticed with an exception at 5KGy dose where the intensity has been found to increase. Further the intensity variation is confirmed by calculating the G-values corresponding to the absorbance bands. |
References |
|