ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Shital N. Bhad1*, V.S.Sangawar2 ,A.K.Maldhure3 ,D.P.Tayade4, G.R. Yerawar5
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
PVA gel electrolyte with different concentration of PVA (0.5, 1 ,1.5, 2 ,2.5 by weight) in KSCN+DMSO electrolyte has been prepared by simple technique and characterized by X-RD and measuring d.c. conductivity electrical conductivity as a function of temperature in the range 00to 1000c . Variation of conductivity of gel electrolyte with loading concentration of PVA has also been studied .It has been observed that all gel sample are amorphous in nature with shifting a broad peak. As loading of PVA in 0.2 M electrolyte of KSCN+DMSO increases conductivity decreases. But conductivity is found to be increases with increases in temperature for all sample consisting VTF and Arrhenius behavior.
Keywords |
| polymer gel electrolyte, ionic conductivity, solvent free polymer electrolyte. |
INTRODUCTION |
| In polymer gel electrolytes the salt generally provides free/mobile ions which take part in the conduction process and the solvent helps in solvating the salt and also acts as a conducting medium whereas the polymer is reported to provide mechanical stability by increasing the viscosity of electrolyte [1]. The possible interactions between the polymer and salt/solvent have not been clearly understood. The salt used should generally have large anions and low dissociation energy so that it easily dissociates whereas the solvent used should have high dielectric constant, low viscosity and high boiling point and low melting point. The addition of polymer to lithium ion conducting polymer gel electrolytes has been generally found [2] to result in an increase in viscosity due to which mobility decreases and as a result conductivity also decreases (s =nqm), whereas in the case of proton conducting polymer gel electrolytes containing weak acids the addition of polymer has been reported [3-7] to result in an increase in conductivity. |
| In recent years, polymers such as polyvinyl alcohol (PVA) poly (vi-nylidene fluoride) (PVdF), Poly ethylene glycol (PEG), poly(vinyl chloride) (PVC), poly(acrylonitrile) (PAN), poly(vinyl pyrrolidone) (PVP) and poly(vinyl sulfone) (PVS) have been prominently used in development of GPEs [8-11]. In gel polymer electrolytes (GPEs), which have both solid and liquid like properties,[12][13]. These electrolytes have been found to possess ionic conductivity, electrochemical stability and transport properties similar to their liquid counter parts along with better dynamical properties suitable for electrochemical applications. Among the listed polymeric hosts, Poly (vinyl alcohol) has been intensely investigated .Poly(vinyl alcohol) (PVA) appears to be very attractivematerial for preparing proton conducting polymer gel electrolyte because this polymer can function as an excellent methanolbarrier.[14] PVA also has both very good mechanical propertiesand chemical stability, which are adequate for preparing proton conducting polymer gel electrolyte.[15] |
| In the present investigation, an attempt has been made to synthesize PVA based gel electrolyte with potassium thiocyanateand itsX-RD and d.cconductivity. |
EXPERIMENTAL |
| A. Chemical: |
| In the present investigation polyvinyl alcohol, potassiumthiocyanate (KSCN), and aprotic solvent dimethyl sulfoxide(DMSO) AR grade, s.d fine chem. Make were used for synthesis of proton conducting |
| B. Synthesis: |
| For the synthesis of PVA gel 30 ml electrolyte of 0.2 M solution of KSCN+DMSO was prepared. PVA was added in the electrolyte with a constant stirring at constant temperature 700C and stirring was continued for one hours. Then it was allow to cool below room temperature for 6 hours , so as to form the gel. PVA has been added in the electrolyte in different amount 0.5, 1, 1.5, 2, 2.5 by weight to form the gel of various concentration of PVA. |
| C. Measurement technique: |
| Structural morphology of PVA gel electrolyte have been carried out by X-ray diffractometer.The conductivity of PVA gel electrolyte was measured by 304 systronicconductivity Bridge. |
RESULT AND DISCUSSION |
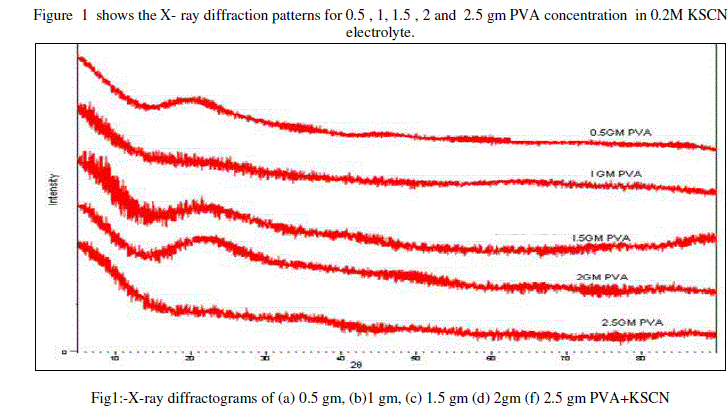 |
| X-ray diffractogram do not show any sharp peak, indicates that the amorphous of the samples of gel electrolyte. The broad peak is observed for 0.5 gm at 200 and 1.5 gm, 2 gm PVA at 220. All gel samples shows an amorphous nature it might be due to proper mixing of ingredients PVA, KSCN, DMSO.[16]The slight shifting of peaks is observed, this shifting of PVA gel related peaks can be co-related to complexation of pristine components leading to the formation of polymer gel electrolyte.[17][18] |
| D.C. CONDUCTIVITY: |
| Figure 2 depicts the variation of conductivity with concentration of PVA (in gm) in KSCN + DMSO liquid electrolyte at room temperature observed that conductivity decreases as concentration of PVA increases. This can be explained as follows, |
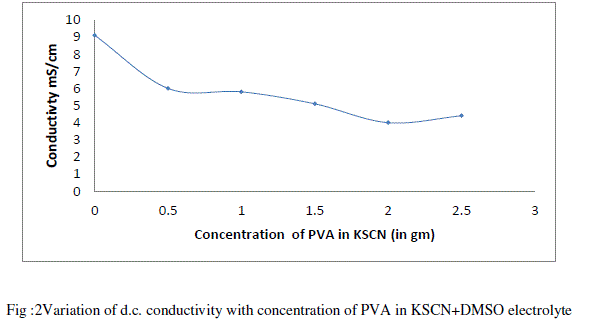 |
| As loading of PVA in liquid electrolyte increases viscosity of electrolyte increases. Due to increase in viscosity number of dissociated charge carrier decreases which further decreases the ionic mobility and hence conductivity decreases. [19][20]. When concentration of polymer increases there is a formation of ion aggregation. These ionic aggregates impede the conduction process and decreases the conductivity [21][22] Figure 3 depicts the temperature dependence conductivity of 0.2 M KSCN electrolyte and PVA gel electrolytes. From fig it is observed that for liquid electrolyte and all the sample of PVA gel electrolyte. |
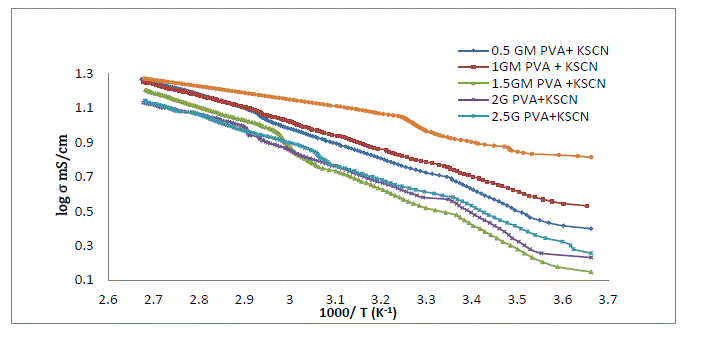 |
| The conductivity of liquid electrolyte increases with increase in temperature this might be because of dissociation of charge carrier in liquid is a consequence of increasing the concentration of mobile ion with temperature is unlikely[23]Conductivity]Initially there is a large difference in conductivity of liquid electrolyte and polymer gel electrolyte. As the temperature increases conductivity of gel electrolyte increases linearly also the difference in conductivity value in liquid electrolyte and polymer gel electrolyte goes on decreasing .At higher temperature there is a slight variation in conductivity of liquid electrolyte and polymer gel electrolyte. At high temperature the variation of conductivity of gel electrolyte is similar to that for liquid electrolytes, which also suggest a liquid like behavior of gel [24] |
| The curve for all the gel samples shows three different region .Also rate of variation of conductivity is different in different temperature region. The conductivity increase with temperature and follows Arrhenius behaviour and VTF behaviour through the region [25] |
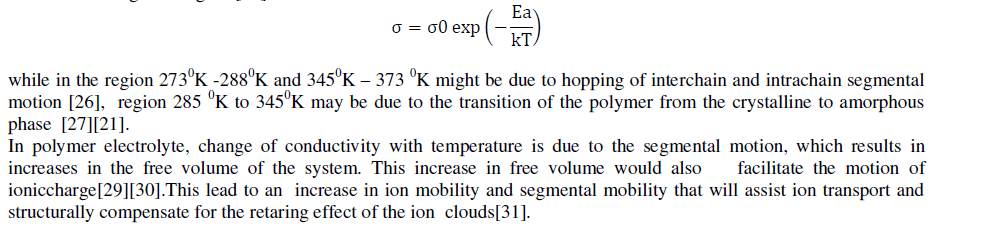 |
CONCLUSION |
| Gel electrolytes prepared by addition of PVA 0.2 M KSCN in DMSO have been structurally and electrically characterized in the present work. . XRD patterns exhibit broadening of peak in diffraction patterns of PVA gel electrolyte indicating increase in amorphousness. Gel of higher concentration of polymers shows the presence of PVA: KSCN complex and conductivity is seen to be influence by the presence of this complex .Temperature dependence of ionic conductivity of gel electrolyte reflect Arrhenius and VTF behaviour. |
References |
|