ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Mohammed1, A.El-Maghraby2,3
Related article at Pubmed, Scholar Google
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Composition of Sn99.5-xCu0.5Bix has been examined as one of the lead free solder alloys and considered for soldering in electronic packaging. The addition of bismuth to Tin improves the ductility and restrains the fillet lifting, which are problems of lead-free solders with Bi. Melting point of the samples were studied; the addition of Bismuth to SnCu0.5 binary alloy lowers the melting temperature. Wetting contact angle measurements of the Sn99.5 Cu0.5, Sn59.5 Cu0.5Bi40, Sn54.5 Cu0.5Bi45 and Sn49.5 Cu0.5Bi50 rapidly solidified lead free solder alloys on CuZn30 substrate were carried out at 573 K. The internal friction, elastic moduli, microhardness and the microstructure of the as-cast alloys at room temperature have been investigated. The examined physical properties are improved by increasing bismuth contents in the studied lead free solder alloys. These improved properties indicate that these alloys are adequate for low temperature soldering applications
Keywords |
| Solder alloys, Young’s modulus, microhardness and wetting |
INTRODUCTION |
| Soldering technology is the most important joining technology in the electronic industry and has been widely used for the joining of the modules (first level package) to printed circuit boards (PCBs). The soldering technology is now becoming even more important because of the fast growing usage of the ball-grid-array (BGA) package and flip-chip technology [1]. For global environment conservation, many active researches on replacing the conventional SnPb eutectic solder with a Pb-free solder have been carried out. Among lead-free solder materials, Sn-Ag alloys appear to be promising substitutes due to their high joint reliability. However, Sn-Ag alloys are not easily applied to some electronic components, which have low-heat-tolerance because of the high melting temperature of these alloys. As another alternative, lead-free solders Tin-Cupper alloys with different melting points are often used in the step soldering technology, where soldering is applied more than once during the manufacturing. Addition Bi offers a low-melting temperature,which enable a lower soldering temperature, and have high tensile strength. These advantages have attracted a great deal of attention to Pb-free solders containing Bi. Adding Bi to the alloy, however, makes the solder less ductile [2]. In low temperature microelectronic package technology, the most potentially suitable alternatives are SnBi solders due to their lower melting temperatures. The melting point of eutectic SnBi58 solder is 411 K, while the eutectic temperature of SnAg3.5 solder is 494 K [3], good wettability, relative good yield strength (YS) and ultimate tensile strength (UTS). Moreover, Miao et al. [4, 5] reported that the microstructure coarsening was substantially reduced by an addition of 1wt % Cu into the binary SnBi solder. Hence, SnBiCu ternary solders have better market potential than SnBi solders in low temperature microelectronic package industry. Therefore, Bi were chosen in the present work as alloying element to SnCu0.5 alloy in order to improve the soldering property in electronic packaging by lowering melting point. |
II. EXPERIMENTAL |
| The solder alloys with compositions as listed in table (1) were prepared from spec-pure elements (better than 99.9% in weight percent).The materials of pure Sn, Cu and Bi were weighted and melted in a porcelain crucible at 500 oC for 1 h. From these alloys, long ribbons were prepared as the test samples by directing a stream of molten alloy onto the outer surface of a rapidly revolving aluminium roll. The speed of the aluminium wheel was fixed at 1820 r.p.m. |
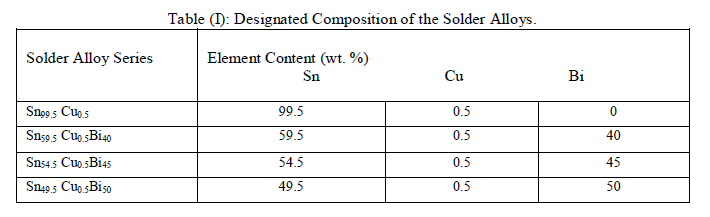 |
| The melting points of the solder alloys have been analyzed by differential thermal (DTA). Melting temperature of the samples was measured with scanning temperature range from 50 to 300 oC at a heating rate 10oC/min. A (CuZn30) substrate with solder balls of weight of 1 g were prepared for measuring the wettability property of the SnCuBi solders alloys. The contact angles were recorded photographically at 573 K. The substrate were immersed in a solution of 30% HCL and 70% distilled water for degreasing, followed by cleaning in ethanol and drying in air. The internal friction and dynamic young’s modulus of melt-spun alloys was examined by a dynamic resonance method. Microhardness tests applying a load of 25 gm for 20 s by a Vickers diamond pyramid [6]. Five readings of different indentations were taken at room temperature to calculate the mean value and the standard deviation. To disclose the effect of adding Bi on the microstructure of the rapidly solidified SnCu solder alloys, the author analyzed these samples using scanning electron microscope (SEM). All samples etched in a solution consisting of (3 ml HNO3 + 97 ml alcohol) for about 10 s and washed by distilled water. In this paper, it is anticipated that addition of Bi can improve the mechanical properties of Sn99.5 Cu0.5 alloys. |
III. RESULTS AND DISCUSSION |
III.1.DENSITY |
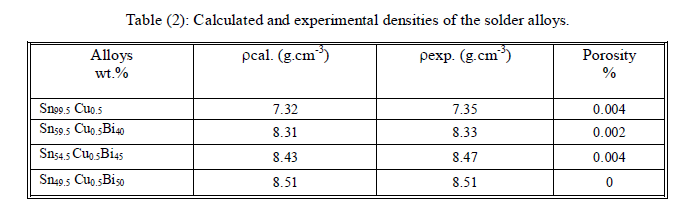
| The density (ïÃÂò) of each alloy sample was measured by the displacement method using carbon tetrachloride (CCl4) as the immersion liquid. The experiment was repeated three times, the calculated and experimental density measurement for all alloy samples as shown in table (2). |
 |
 |
III.2. THERMAL ANALYSIS |
III.2.1.DIFFERENTIAL THERMAL ANALYSIS |
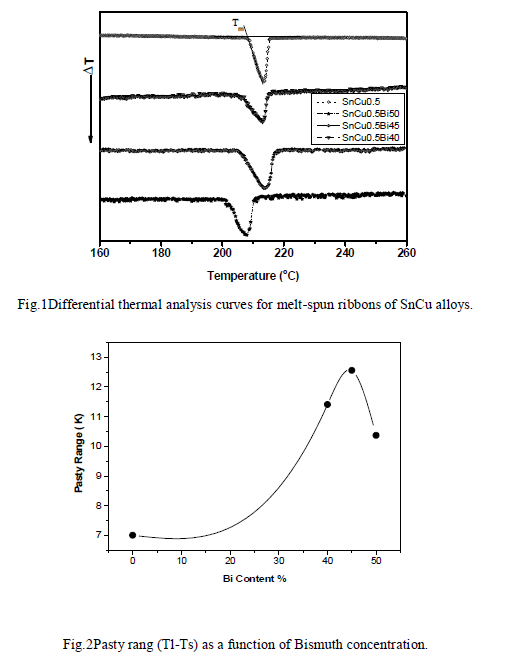
| Figure (1) shows the DTA curves for Sn99.5 Cu0.5, Sn59.5 Cu0.5Bi40, Sn54.5 Cu0.5Bi45 and Sn49.5 Cu0.5Bi50 solder alloys. It shows that there is no any phase transformation observed in the temperature range from 50 to 300 oC except the melting, which is indicated by the endothermic peak of melting for all alloys. The onset point of endothermic peak is the starting temperature of a reaction and the offset point is the end temperature. On heating, the onset point is called the solidus temperature of the alloys and the offset point is called the liquidus. The pasty range (P.R) is the differences between the liquids and solidus temperatures. The solder is semisolid in the pasty range. The addition of Bi to the Sn99.5 Cu0.5 alloy system expands the pasty range in the melting reaction. The plots of the transformation points on heating as a function of Bi content are shown in Fig. (2). The transformation temperatures of the alloys decrease with increasing of Bi content. The onset point and the offset point decreases with the increase of Bi content from 208 at Sn99.5 Cu0.5 alloy into 216.8oC at Sn49.5 Cu0.5Bi50 alloy and from 201.9 into 210.7oC respectively. |
 |
III.2. 2.WETTING PROPERTIES OF SOLDERS |
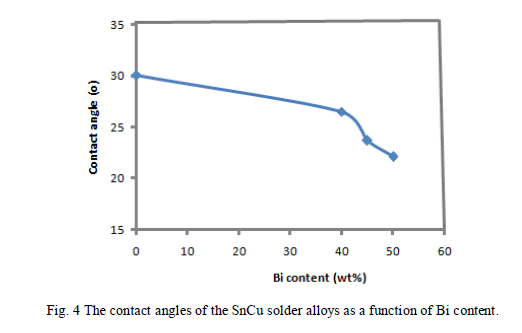
| Few experimental works on the wetting properties of the Sn-based alloys (Sn–Pb, Sn–Bi, Sn–Ag, Sn–In, Sn–Cu, Sn– Ag-In) on copper substrates are reported in the literature [7,8 and 9]. In particular, Arenas and Acoff. [8] have measured the contact angle of Sn–0.7 wt % Cu on a Cu substrate and obtained a value around 35o at 513 K. The contact angle of four Sn-rich alloys (Sn99.5 Cu0.5, Sn59.5 Cu0.5Bi40, Sn54.5 Cu0.5Bi45 and Sn49.5 Cu0.5Bi50) was measured on a CuZn30 substrate in a temperature range from the liquidus temperature up to 573 K. the relationship between contact angle of wetting and time was recorded as the wetting curve as shown in Fig. (3). |
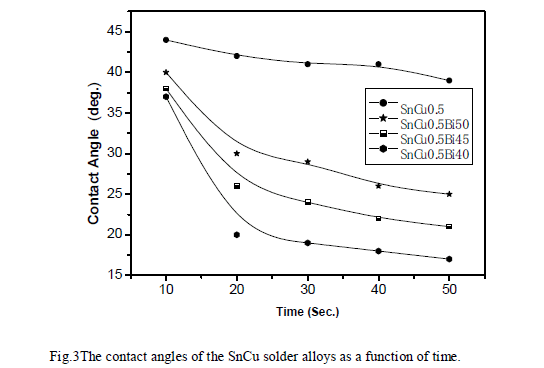 |
| Figure (3) indicates that the wetting properties of the solder joints improve with Bi addition, its wetting force increased and the wetting time reduced, and achieve the optimal wetting performance when the Bi content is 40 wt.%. The effect of Bi in improving the wetting behavior of SnCu solder alloy is better than that in SnAgCu solder [10]. The contact angle measurements were performed on rapidly solidified SnCu alloys on CuZn30 substrate at 573K. The contact angle data indicated that Bi additions to the SnCu binary alloy resulted in decrease in the contact angle values from 30o for the Sn99.5 Cu0.5 alloy to 22.1o for the Sn59.5 Cu0.5Bi40 alloy as shown in Fig. (4). Adding Bi into Sn99.5 Cu0.5 alloys are shown to be some of the best choices lead-free solders [11]. |
 |
III.3.MECHANICAL PROPERTIES |
III.3.1.MICROHARDNESS MEASUREMENTS |
| Resistance to plastic indentation was determined by an indentation hardness test; in which small hard indenter is pressed into the surface by a standard load and the size of indentation produced by it is then measured. As seen from the table (3), the variation of microhardness Hv with Bi addition (Wt %). The average hardness values, Hv increase significantly with increasing amount of bismuth, indicating that the bonding force between bismuth and Cu or tin is much stronger than that between Cu and tin. Also such variations in bismuth content causes an increase in hardness value are generally taken as indicative of in homogeneity of the system. Bi was reported to increase the matrix hardness up to 5 wt% which is about the solubility limit of Bi. |
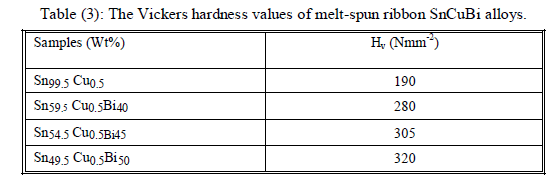 |
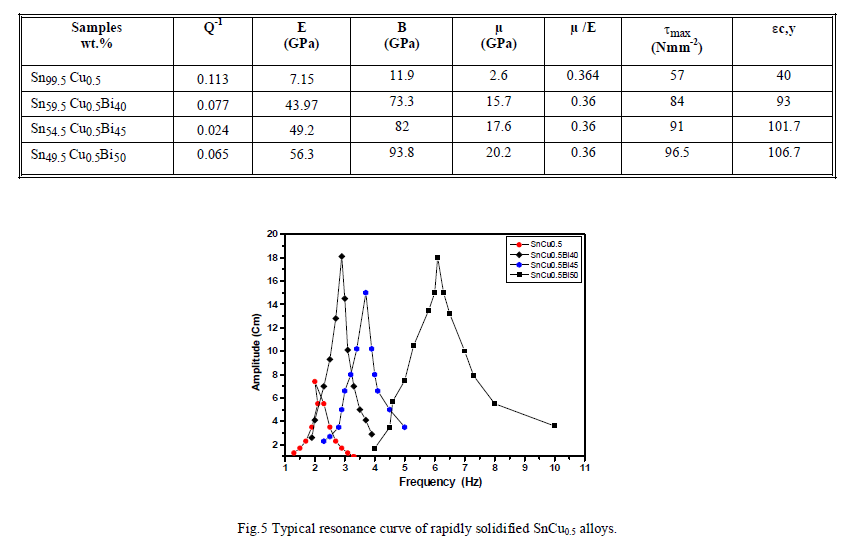
| III.3.2.INTERNAL FRICTION AND DYNAMIC YOUNG’S MODULUS Figure (5) shows the resonance curves for melt-spun ribbons of Sn99.5 Cu0.5 alloys. Table (4) shows the variation of the dynamic Young’s modulus E with the variation of different compositions of Bi content. As first, the value of E for Sn99.5 Cu0.5 alloy found to be (7.15GPa). After the addition of Bi, the value of E increases to maximum value (56.3GPa) for Sn49.5 Cu0.5Bi50 alloy. This observed behavior of Young’s modulus with Bi content due to the presence of hard intermetallic compounds, which act as hard inclusions in the soft matrix. The measured internal friction Q−1 values are listed in table (4) as function of composition. It is concluded that the internal friction is sensitive to the content of Bi as alloying element to the binary Sn99.5 Cu0.5. It is shown that the internal friction Q−1 decreased with the addition of Bi is explained by a reduction in transformable shear stress with largest free volume. Generally high damping capacity is practical engineering importance in limiting the amplitude of vibration at resonance conditions and thereby reducing the likelihood of fatigue failure. Table (4) shows the values of the bulk modulus (B) and shear modulus (ïÃÂÃÂ) of Sn99.5 Cu0.5, Sn59.5 Cu0.5Bi49, Sn54.5 Cu0.5Bi45 and Sn49.5 Cu0.5Bi50 rapidly solidified alloys, respectively. This is calculated using the dynamic resonance method; the measured results indicate that the elastic modulus value of Sn99.5 Cu0.5 increased by the addition of Bi. These results showed that, the addition of Bi content cause a marked change in the growth morphology such as dimensions, distribution density and shape of SnCu compound [12]. Generally, the addition of Bi to SnCu alloy cause to form intermetallic phases such as BiSn and CuBi. Table (4) shows that the value of μ/E of the rapidly solidified solder alloys are in good agreement with the commonly accepted values that were derived theoretically according to Ledbetter and Zwikker (μ/E = 0.357) [13, 14]. The maximum shear stress, τmax. which is produced by a locally applied pressure, occurs on the central axis below the pressurized region [15, 16]. This will be given by the following equation. τmax. = 1/2HvïÃÂû1/2(1-2ïÃÂç)+2/9(1+ïÃÂç)ïÃÂÃâº2(1+ïÃÂç)ïÃÂÃÂ1/2ïÃÂý (1) where ïÃÂç is Poisson’s ratio and its value is 0.4, this gives τmax. = 0.31 Hv (2) Shear stress is a measure of the resistance to separation of adjacent atoms, that is, the interatomic bonding force [10]. By increasing the bismuth concentration, the shear stress τmax increase to the maximum value (96.5 Nmm-2) at Sn49.5 Cu0.5Bi50 alloy as shown in table (4). Table (4): Internal friction (Q-1), Young’s modulus (E), bulk modulus (B), shear modulus (μ), shear stress (ïÃÂômax) and calculated compressive yield strain of melt-spun ribbon SnCuBi alloys. |
 |
| The calculated compressive yield strain (ïÃÂÃÂ¥c,y= Hv/3) are shown in table (4). It is found that the compressive yield strain increase with addition of Bi (wt %). This may be due to high performance metal alloys are never single phase but generally consists of a matrix interspersed by areas of an intermetallic phases. III.4.MICROSTRUCTURE According to the SnBi phase diagram [11] considered from the multiphase alloys whose chemical composition does not correspond exactly to the eutectic concentration, a precipitated phase can segregate along with the eutectic. For instance in the alloys whose concentration is within the temperature interval between the liquidus and solidus lines [17], a precipitated phase (ß phase) usually solidifies. This phase is in the form of large grains that are linked to the peculiarities of the growth of the precipitated phase as solidification of the alloy proceeds within the temperature interval between the liquidus and solidus. Figure (6) shows the scanning electronic microscopy image of microstructures of as-cast SnCuBi solders alloys. The structure of cast tin bronze containing Sn99.5 Cu0.5 is illustrated in Fig. (6a). It consists of alpha solid solution of copper in tin and eutectic (alpha + ïÃÂÃÂ¥) [18]. In addition to the grains of alpha solid solution (or ïÃÂá- solid solution and secondary precipitates of Cu3Sn). They are formed due to the strong segregation and low rate of the diffusion processes [19].Large needle-like Cu-rich phase distributed irregularly in the Sn matrix. With the addition of 40 – 50 % Bi to the SnCu0.5 alloy, the volume fraction and size of the needlelike phase tends to be decreased, and obtain finest and uniform structure when 40 wt% Bi is added, as show in Fig. (5b to d). According to the grain-refining strengthening theory [19], the strength of the alloy will increase significantly with Bi added. Its mechanical properties are expected to be improved too. When Bi content is up to 40 %, some light particulate-shaped phases can be observed in the Sn-rich matrix. These light phases are determined as the Sn-Bi intermetallic compounds as shown in Fig. (6). |
 |
IV. CONCLUSION |
 |
References |
|