ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Ashwini Jagtap1 & Rajendra Mali2 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Temperature is the dominant ecological factor on all animal lives. The aquatic animals are highly sensitive to the temperature fluctuations. In present work, snake headed fish Channa punctatus was exposed for 24, 48, 72 and 96 hours under stress conditions of temperature. The notable changes in total lipid content were observed and compared with control set. The present investigation showed depletion in the total lipid content was observed in liver tissue of freshwater fish, Channa punctatus under cold and warm temperature stress. The total lipid content expressed as percentage of lipid content per gm wet wt. of tissue
Keywords |
| Temperature, Total Lipid, Liver, Channa punctatus |
INTRODUCTION |
| Temperature is an important ecological factor which affects the chemical composition of various tissues. There is a tight relation between the organism and its environment. Each and every organism faces the problem of environmental changes. To overcome this problem the animal tries to adapt themselves against environmental fluctuations. This process of temperature acclimation seen in poikilothermic animals adjusts their metabolic rate in order to maintain physiological activities at a more nearly constant level over a range of environmental temperatures. Thus, they attain a measure of independence of temperature by Prosser and Brown, 1961 (13). Lipids are diverse group of compounds many of which function as important sources of metabolic energy. Lipids found in foodstuffs and in the fat deposits of many animals in the form of triglycerides which are esters of fatty acids and glycerol were investigated by McDonald et.al, 1989 (8). They contain more energy per unit weight than any other biological compound. They are important in maintaining structural and physiological integrity of cellular and sub-cellular membranes. They provides a source of indispensable nutrients and act as carriers of certain non fat nutrients, notably the fat soluble vitamins like A, D, E and K by various researchers viz., New, 1986 (10); Jauncey, 1988 (6); Richardo et al. 2003 (16). The increase in temperature affects on the life of animals studied by Frumhoff, et. al., 2007 (4). Solmon et. al., 2007 (5) studied on the global climate changes and its effects on organisms. Brown and Rose, 1969 (2) carried research on the correlation between dissolved oxygen and cold and warm temperature; its effects on the total lipid contents. Bernd et. al., 2006 (1) shows the effect of low temperature on the lipid contents in poikilotherms. The physiology of poikilotherms in relation to temperature stress on the total lipid contents were observed by Normon, 1990 (11) on warm acclimated fish, Lesile, et. al., 1976 (7) on Goldfish, cold water specie by Oliveira, et.al,. 2003 (12), Nargis, R. 2006 (9), Rao, 1996 (14). |
| The frequency of changes in the composition of biochemical constituents of any organism varies with the fluctuations of the environmental changes. Biochemical studies are good parameters which help to see the effect of temperature on biochemical composition of vital tissue of fish. Hence attempt has been made to find out biochemical changes in total lipid content in Liver of freshwater fish, Channa punctatus. |
II. MATERIAL AND METHODS |
| The freshwater fish, Channa punctatus was collected from the Godavari River, Nanded (Maharashtra). They were brought to the laboratory and kept in glass aquarium with continuously aerated tap water. The fishes were acclimated at room temperature for 8-10 days prior to experimentation. Fishes were feed with the small pieces of earthworms. The water in the aquarium was replaced daily with fresh tap water. The fishes were subjected to above and below room temperatures to carry out experiment. The four experimental sets were designed to carry out experiments (two sets at below room temperatures and two sets at above room temperatures). The animals were divided into five groups having 10 fishes in each aquarium according to Fletcher, 1977 (3). The fishes were acclimated to different temperatures for 24 hrs, 48 hrs, 72 hrs and 96 hrs. Healthy uninjured fishes ranging from 60- 70 grams were selected for present study. Estimation of Lipid was carried out by Methanol-Chloroform Method by Raymont et. al., 1964 (15). The total lipid content expressed as percentage of lipid content per gm wet wt. of tissue. |
III. RESULTS |
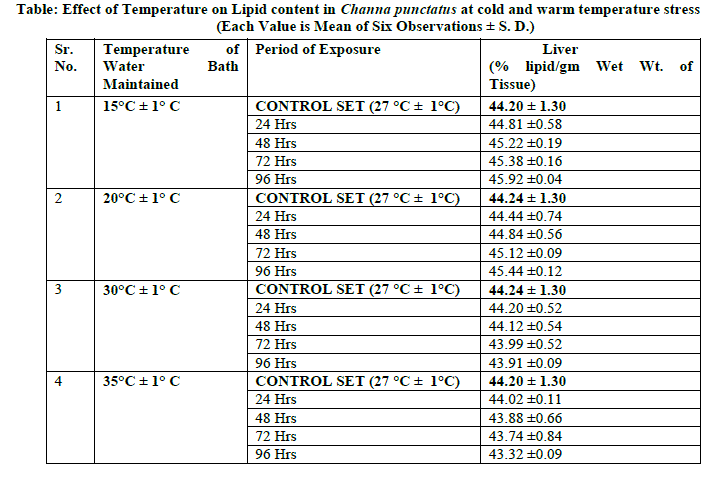
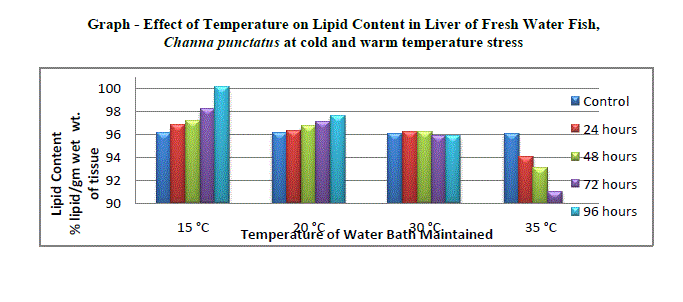
| Effect of temperature on snake headed freshwater fish Channa punctatus causes changes in Lipid content when acclimated to cold and warm temperature stress along with control. The results of lipid content in liver of fresh water fish in relation to thermal stress 15° C, 20° C, 30° C and 35° C have been represented in Table and the graph was plotted in Figure; the values were expressed in % lipid/gm wet wt. of tissue. The lipid content in liver of fresh water fish Channa punctatus control set was found 44.20 % lipid/gm wet wt. of tissue. The fish exposed to cold temperature stress showed increase in lipid content in liver at 15 ± 1° C. The slowly increasing trend was observed at this temperature up to 96 hrs periods of exposure (44.81, 45.22, 45.38 and 45.92 % lipid/gm wet wt. of tissue) relative to that of control set (Graph). At 20 °C the increasing trend was continues at this temperature stress up to 96 hrs period of exposure as compared to control set. The lipid content in liver of control set was found 44.24 % lipid/gm wet weight of tissue and that for 24 hrs, 48 hrs, 72 hrs and 96 hrs period of exposure were found to be 44.44, 44.84, 45.12 and 45.44 % lipid/gm wet wt. of tissue respectively (Graph). The recoded value of lipid content for control set at 30 °C lipid in liver was 44.24 % lipid/gm wet wt. of tissue. The freshwater fish, Channa punctatus when acclimated to 30 ± 1° C showed declined in lipid content up to 96 hours period of exposure as compared to control set. The values obtained for 24 hrs, 48 hrs, 72 hrs and 96 hrs period of exposure were found to be 44.20, 44.12, 43.99 and 43.91 % lipid/gm wet wt. of tissue respectively (Graph). |
| The amount of lipid in liver of ambient temperature was 44.20 % lipid/gm wet wt. of tissue. The freshwater fish, Channa punctatus when exposed to warm temperature stress at 35 ± 1° C showed decreasing trend in total lipid content up to 96 hrs period of exposure as compared to control set. The values obtained for lipid content in liver for continuous exposure up to 96 hrs period of exposure were found to be 44.02, 43.88, 43.74 and 43.32 % lipid/gm wet wt. of tissue respectively (Graph). |
 |
 |
IV. DISCUSSION |
| The present investigation was based on physiological responses in lipid metabolism of Channa punctatus to acute temperature stress, to determine the ability of species to adapt at increasing temperatures whether they will face with climate changes. Climate change is projected to cause atmospheric temperatures to increase by 3-10º C by 2100, which is likely to cause mass species extinction by Frumhoff, et. al., 2007 (4); Solmon et. al., 2007 (5). The present investigation showed depletion in the total lipid content was observed in liver tissue of freshwater fish, Channa punctatus under cold and warm temperature stress. The reduction in total lipids content in liver of fish was may be due to increased metabolic rate results the increase in demand for energy. This causes the energy reservoir- lipid to undergo more oxidation rate for proper supply energy to various tissues. The reduction of lipid content was may be due to reduced oxygen solubility at elevated temperatures may retard the activity of the oxygen-dependent fatty acid desaturases investigated by Brown and Rose, 1969 (2). |
| Lowering the ambient temperature of poikilotherms is likely to cause an increase in membrane lipid order. Because of the mixture of lipid classes and lipid molecular species in biological membranes, this will mean that one or more of these molecular species will be below its transition temperature. As soon as this phenomenon becomes significant then phase separation of the affected lipids can take place. This results in the impairment of membrane functions. So the poikilotherms increase their average membrane lipid order by Bernd et. al., 2006 (1). Norman, in 1990 (11) reported that in liver of the warm-acclimated fish had significantly lower amounts of total lipid content than the cold-acclimated animals. Leslie, et. al., 1976 (7) studied on Phospholipid composition in liver of goldfish (Carassius auratus L.). He was found that the molecular species of phosphatidyl choline produced by liver choline phosphotransferase decreased in unsaturation as the temperature of incubation was increased from 10 to 30°C. This suggests a possible mechanism by which tissue phospholipid fatty acids could be altered in response to changes in temperature. The co-relation between effect of environmental temperature and the utilization of fatty acids and source of metabolic energy between cold water species demonstrated in species by various investigators by Oliveira, et.al,. 2003(12); Nargis, R. 2006 (9). |
| Rao, 1996 (14) reported increase in the unsaturation lipid content by mobilization of saturated triglycerides in cold acclimated rainbow trout. He observed that, cold acclimation leads to an incline in phospholipids, ketone bodies, cholesterol and lipase activity. |
References |
|