ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Hadi .Z.Al-Sawaad1 Mooslim S. Jabar1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In this study Metronidazole drug is used to reduce the conductivity of 0.5M hydrochloric acid at different concentrations for each one of them at different temperatures ranged (30-60)°C. Generally , at constant temperature as concentration of Metronidazole increased as the inhibition efficiency increased due to the reduced in molar conductance. On the other hand, at constant concentration for the inhibitor, as temperature increased, the inhibition efficiency increased where, in both cases the ionic mobility of the acid is reduced i.e., the molar conductance of the acid is reduced in presence of the inhibitor compared with the absence of it. i.e., Metronidazole can adsorbed chemically on the metal or alloy. Furthermore the kinetic study of the molar conductance process reveal that in presence of Metronidazole the activation energy and enthalpy of activation are negative compared with their values in absence of paracetamol where they are negative because the reducing the conductivity in presence of Metronidazole where the non spontaneous property for the conductance of acid is increased as the Metronidazole concentration increased in addition to increasing the negative value of entropy in presence of Metronidazole that indicate to restrict for the mobility of hydrogen and chloride ions which correspond to 94.38% as inhibition efficiency of reducing the conductance of acid by Metronidazole . On the other hand thermodynamic study is achieved which explained the adsorption mode of Metronidazole depend on the temperature where, different modes for the adsorption iso therms can be obtained at each temperature in this study.
Keywords |
| Flagyl, Metronidazole, molar conductance, adsorption isotherms, corrosion Inhibitors, Hydrochloric acid. |
I. INTRODUCTION |
| Metronidazole(Flagyl) is a Metronidazole antibiotic medication used particularly for anaerobic bacteria and protozoa. Metronidazole is the commercial name for 2-methyl-5-nitro - imidazole -1-ethanol having molecular formula C6H9O3N2. Molecular weight of the Metronidazole is 171.16. It is one of the azoles group of anti-microbial agent which is effective against anaerobic microorganisms including gram positive, gram – negative, cocci and bacilli [1-2]. Electrical conductivity is one of the principle transport properties of aqueous electrolyte systems. In the area of corrosion protection , electrical conductivity provides useful information for assessing the corrosivity of aqueous media and for the design of cathodic protection system. Also conductivity is used to again insight into the properties of the electrolytes solutions. The concentration of electrical conductivity has been extensively investigated for dilute aqueous solutions . A limiting law for conductivity was developed by Onsagar by using the Debye-Huckel equilibrium distribution functions[3]. The concentration dependence of molar conductivities indicates that there are two classes of Electrolyte strong and weak electrolytes . The characteristic of a strong electrolyte is that its molar conductivity depends only slightly on the molar concentration. The characteristic of a weak electrolyte is that its molar conductivity is normal at concentrations close to zero, but falls sharply to low values as the concentration increases[4]. A large number of organic compounds have been used as corrosion inhibitors for mild steel and most of them are highly toxic to both human beings and environment. Due to the increasing environmental awareness and the negative effects of some chemicals, research activities in recent times are geared towards developing the less toxic and environmentally safe corrosion inhibitors It has been investigated that some drugs like tryptamine, succinic acid, Lascorbic acid, sulfamethaxazole, cefratrexyl, cefradroxil and alpendazole were effective and eco-friendly inhibitors for acid environments. The inhibitive effect in acidic media through complex formation on metal surfaces by anti-bacterial drugs, namely ampicillin, cloxacillin, flucloxacillin and amoxicillin on controlling corrosion of Al was investigated [5- 7]. the pickling baths are employed to remove undesirable scale from the surface of the metals. Once the scale is removed, the acid is then free for further attack on the metal surface. Hence, several organic compounds containing N, O and S have been studied as corrosion inhibitors by several authors [8,9]. The use of organic compounds with hetero atoms based corrosion inhibitors is often associated with chemical and/or physical adsorption, involving a variation in the charge of adsorbed substance and a transfer of charge from one phase to the other [10]. Special attention was paid to the effect of electron donating atom and electron withdrawing groups which are responsible for adsorption and hence on the performance [11]. Furthermore, it has been observed that the adsorption mainly depends on steric factor, aromaticity and structural properties of the organic compounds, which induce greater adsorption of the inhibitor molecules onto the surface of the metal [12,13]. Synergistic corrosion inhibitor plays an important role both in theoretical and practical research [14-16]. Synergistic inhibition effects of organic inhibitor/metallic ion mixture and organic inhibitors/organic inhibitor mixture on the corrosion metal in acid media have also been reported[17-19]. Evaluation of corrosion inhibitors for mild steel in acidic media is important for both theoretical as well as industrial point of views. Acid solutions are generally used for the removal of rust and scale in industrial processes. Hydrochloric acid is widely used in the pickling, cleaning and descaling of steel and ferrous alloys[20]. Thus, mineral acid solutions such as hydrochloric acid are widely used for various treatments of materials in industry[21,22]. Conductivity is typically measured in aqueous solutions of electrolytes. Electrolytes are substances containing ions, i.e. solutions of ionic salts or of compounds that ionize in solution. The ions formed in solution are responsible for carrying the electric current. Electrolytes include acids, bases and salts and can be either strong or weak. Most conductive solutions measured are aqueous solutions, as water has the capability of stabilizing the ions formed by a process called solvation. As a result, the concentration of ions in solution is proportional to the concentration of the electrolyte added. They include ionic solids and strong acids, for example HCl. Solutions of strong electrolytes conduct electricity because the positive and negative ions can migrate largely independently under the influence of an electric field[23]. Many literature reviews revealed the corrosion inhibitive effect on alloys like mild steel in acidic media. many drugs have been used as corrosion inhibitors, the mechanistic aspects of corrosion inhibition in aqueous media, one of the drugs can be used as corrosion inhibitor in acidic media. The present work is undertaken to evaluate the inhibition effect of Metronidazole in controlling the corrosion of mild steel by evaluating the conductivity of 0.50M HCl in presence and absence of it before using the Metronidazole as inhibitor for the mild steel or any other alloy where, a different concentrations i.e., (10-50)ppm from Metronidazole were prepared as inhibitor for the conductivity of 0.50M HCl at constant temperature. On the other hand, the effect of temperature on the inhibition effect for the drug against the conductivity of the 0.50M HCl at constant concentration was studied at range (30-60) ºC in order to evaluate it as a corrosion inhibitor for steel or other alloy or metals depending on the reducing of the conductivity of the corrosive environment like acid i.e.,0.50M HCl. Thus, the molar conductivity is used in this study because the concentration of electrolyte (corrosive environment) is taken into account. |
II. EXPERIMENTAL PART |
| After the extraction of additives from a Metronidazole by using a methanol as solvent, a stock solution from it (1000 ppm) was prepared by dissolving it (1g) in 1L of 0.50M HCl as a solvent then, a different concentrations from Metronidazole is prepared (10-50)ppm by using 0.50M HCl as a solvent to study their role on the molar conductance of 0.5M hydrochloric acid in addition to study the effect of temperature on the molar conductance of hydrochloric acid in presence and absence of Metronidazole . The structure of Metronidazole show below in Figure 1: |
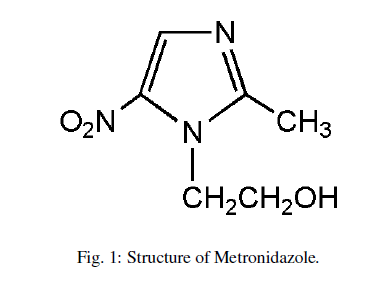 |
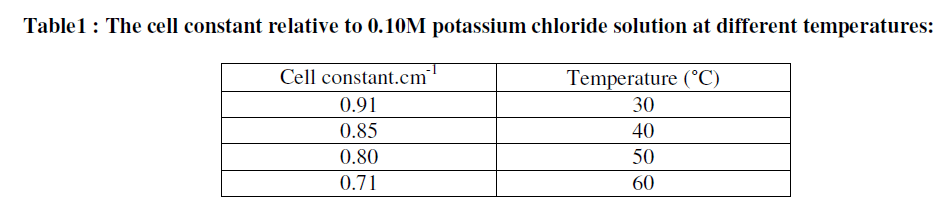
| Then the conductivity of 0.50M HCl is measured in the absence and presence of different concentration of Metronidazole i.e., (10-50) ppm at different temperatures where, the conductivity of distilled water at the temperatures ranged (30-60) °C is subtracted from the conductivity of 0.50M HCl solutions whether in presence or absence of it . On the other hand, the cell constant values of the conductance cell is measured experimentally by using 0.1M KCl solution at different temperatures ranged (30-60) °C as shown in Table 1: |
 |
| Thus, the study of the effect of concentrations of Metronidazole on the molar conductance of the mentioned acid at constant temperature and the reverse can be explained as in results below: |
III. RESULTS and DISCUSSIONS |
| 1. Study the effect of concentration of Metronidazole on the molar conductance of 0.50M HCl at constant temperatures: |
| The conductivity of the 0.5M hydrochloric acid is measured in absence and presence of Metronidazole relative to the conductivity of the distilled water according to the following equation: |
| Where G is the conductivity in Gsolute, Gsolution and Gsolvent are the conductivity in Ω-1 (Semins) for the 0.50 M HCl, the conductivity for the solution and the conductivity for the solvent respectively. On the other hand the specific conductance for the hydrochloric acid solution is measured according to the following equation: |
| Where κ is the specific conductance of the solute in Ω-1.cm-1. Hence, the molar conductance is calculated according to the following equation: |
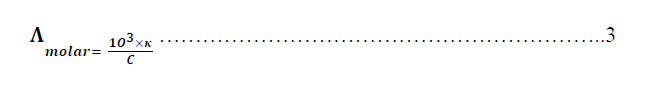 |
| Where Ãâ°Ãâ¦molar is the molar conductance in Ω-1.mol-1 .cm2 and C is the concentration of the hydrochloric acid respectively where C = 0.50M thus, the concentrations of Metronidazole is used to study its effect on the conductivity of hydrochloric acid. In fact, the study of the molar conductance is used due to it express to the real conductivity for electrolyte i.e., hydrochloric acid because, it take into account the concentration of the electrolyte. Thus, the reducing in molar conductivity of the acid can be calculated according to the following equation : |
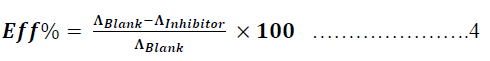 |
| Where efficiency% is the efficiency of Metronidazole to reduce the molar conductance of hydrochloric acid, ΛBlank and ΛInhibitor are the molar conductance of hydrochloric acid in absence and presence of Metronidazole as inhibitor respectively. On the other hand, the ionic mobility can be calculated for the hydrochloric acid according to the following equations: |
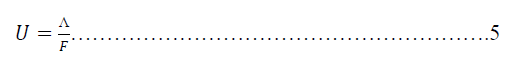 |
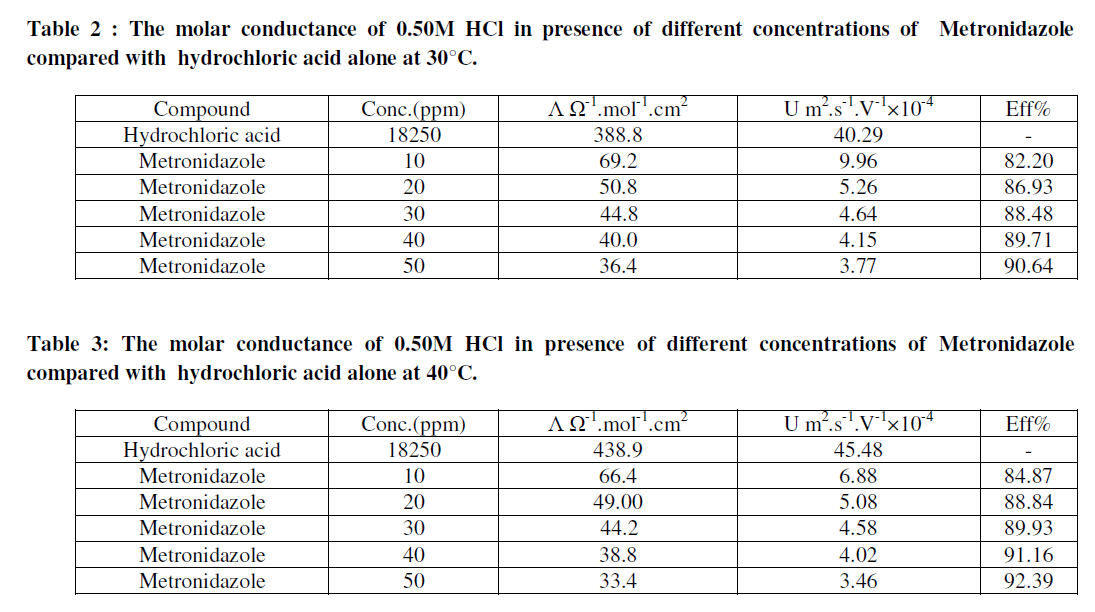
| Where U is the ionic mobility m2.s-1.V-1 for hydrochloric acid and F is Faraday's constant (96500 Coloumb.mol-1). Hence the efficiency and the ionic mobility can be listed as in Tables (2-5) as below: |
 |
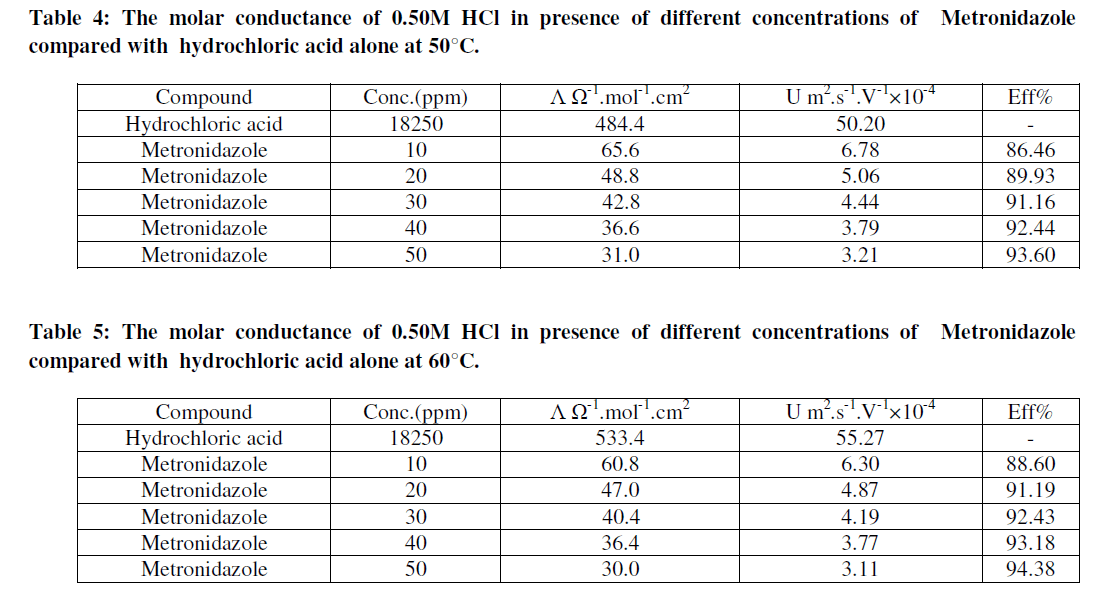 |
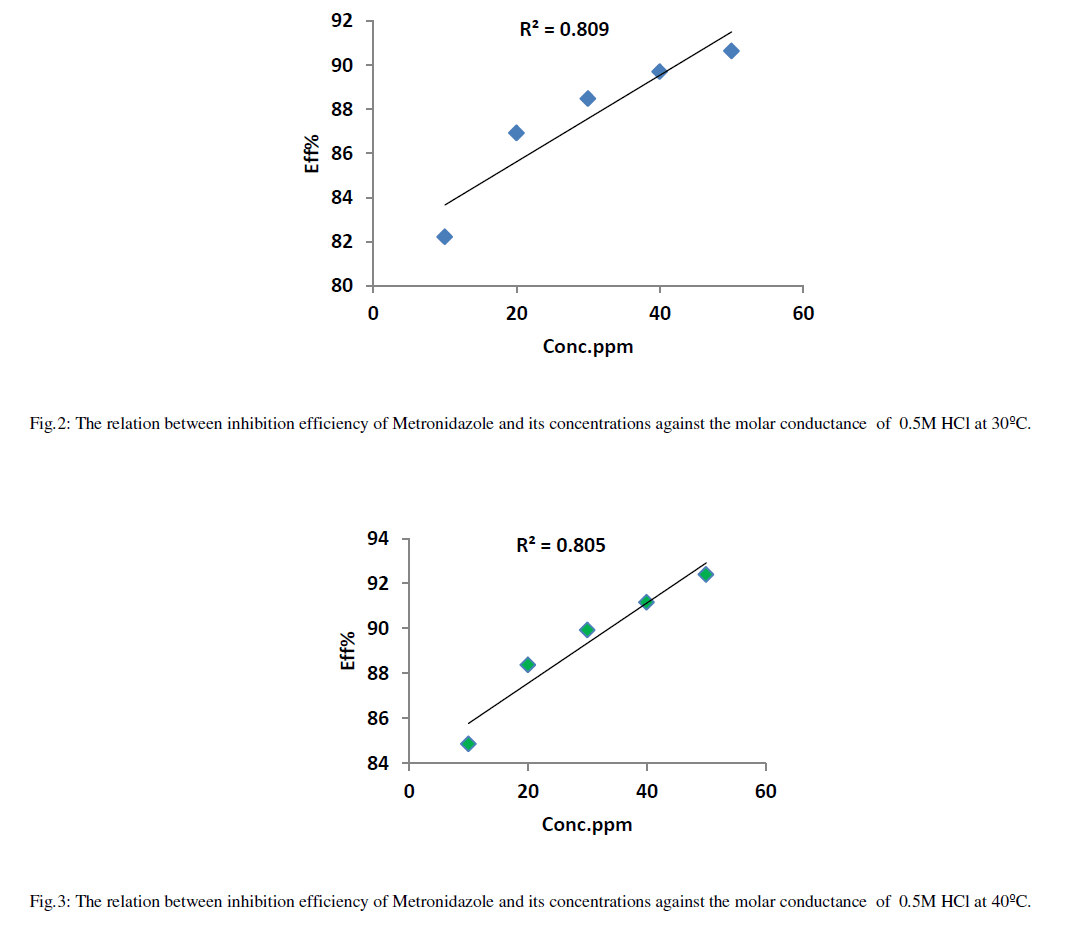
| Tables (2-5) Indicate that both the molar conductance and the ionic mobility are reduced in presence of Metronidazole compared to the presence of 0.50M HCl only taking into account that as concentration of the inhibitor(Metronidazole) increase , the molar conductance and ionic mobility of acid is decreased this can be attributed to the increasing the ability of Metronidazole as its concentration is increased to retard the mobility of the hydrogen and chloride ions for hydrochloric acid toward the their oppose electrodes where the increasing of inhibition efficiency of Metronidazole is increased as its concentration is increased hence, Metronidazole can be evaluated as corrosion inhibitor for a certain metal or alloy due to its ability to reduce the ionic mobility of hydrochloric acid i.e., reducing the evolution of hydrogen gas on the cathode or on the metal surface if immersed in HCl solution. Where the maximum inhibition efficiency at 30°C is 90.64% at 50ppm from Metronidazole compared with other study 84% by using 140ppm from the same inhibitor [2]. The relation between efficiency and concentration of Metronidazole can be shown as in Figures (2- 5) below: |
 |
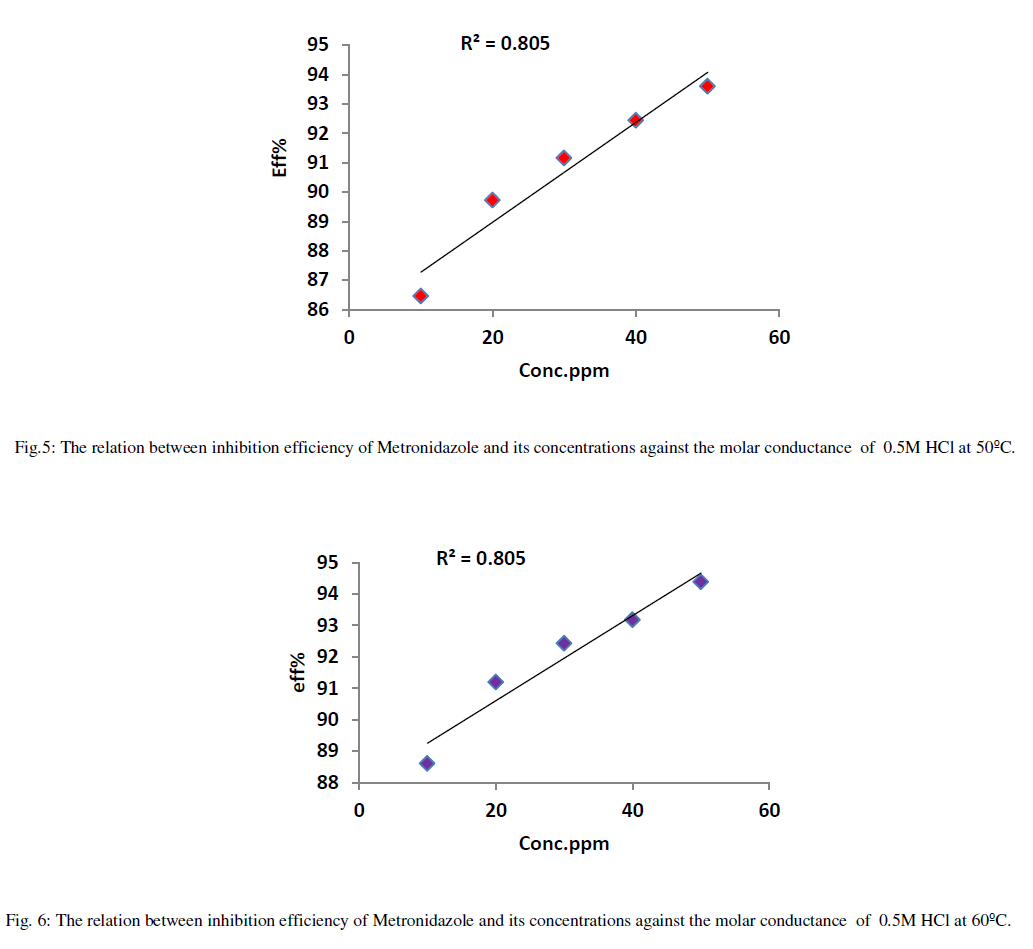 |
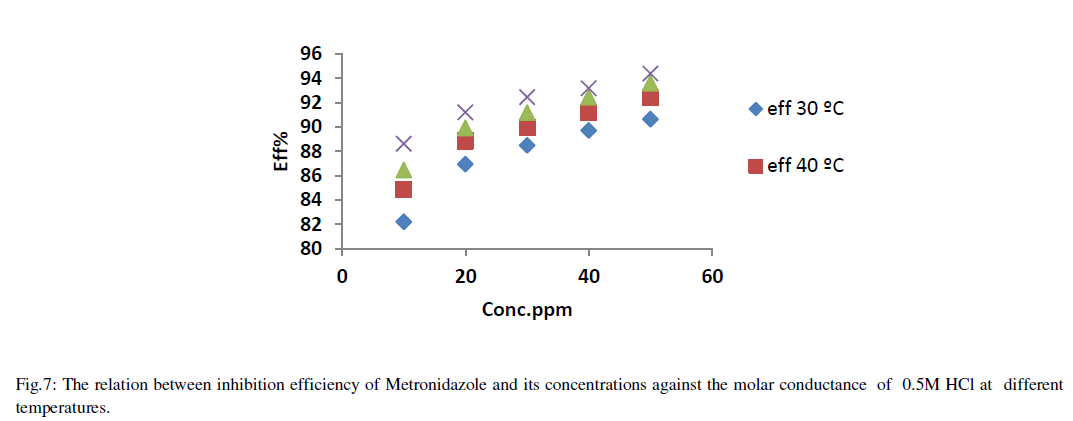
| The relation between efficiency and concentration of Metronidazole a different temperatures can be summarized as in Figure 7: |
 |
| On the other hand, the relation between ionic mobility of hydrochloric acid in presence of different concentration of Metronidazole at different temperatures can be shown in Figure 8 where the zero concentration of it means presence of acid only. |
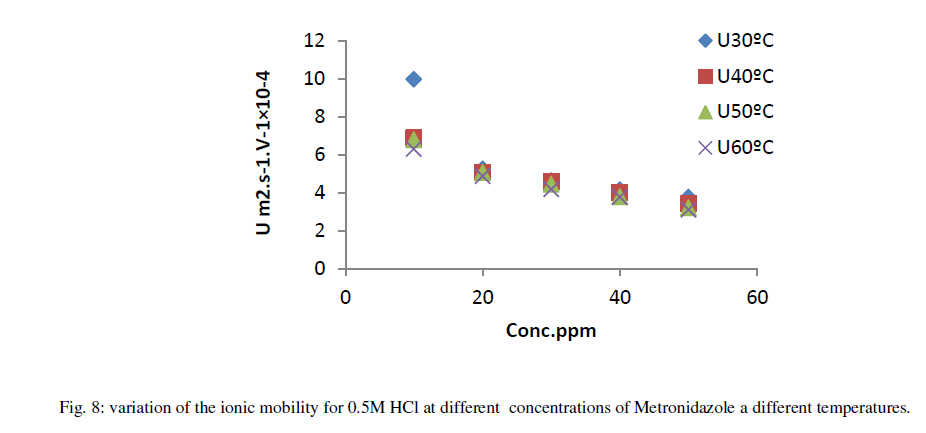 |
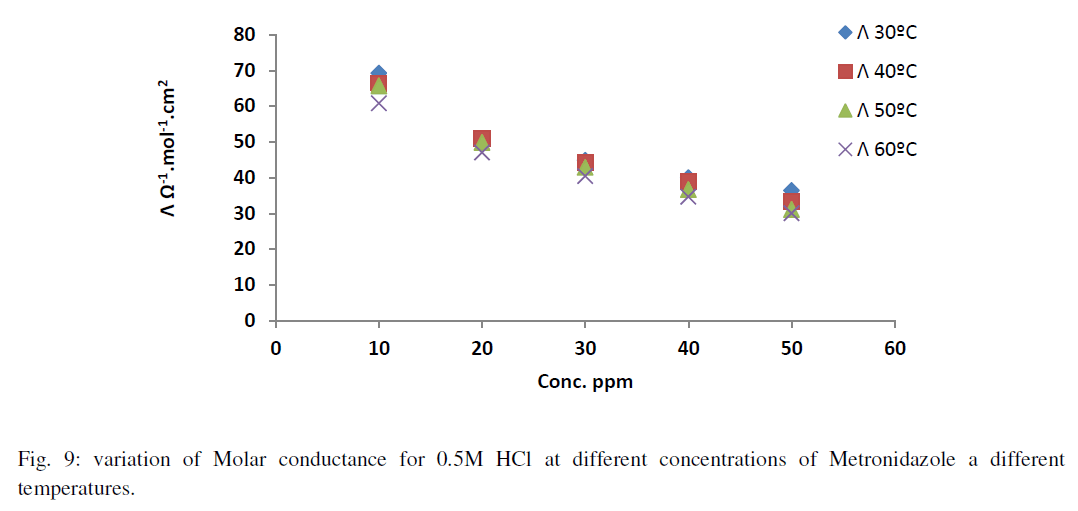
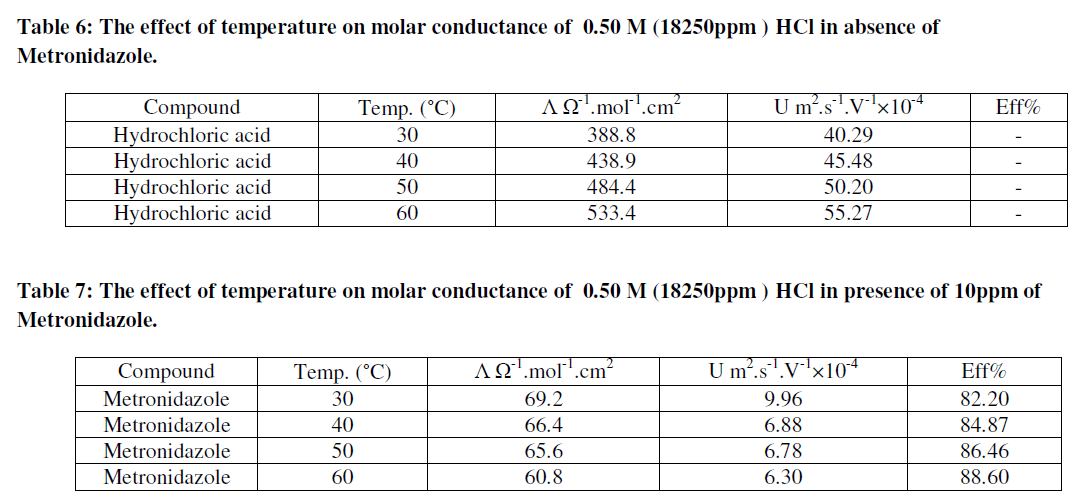
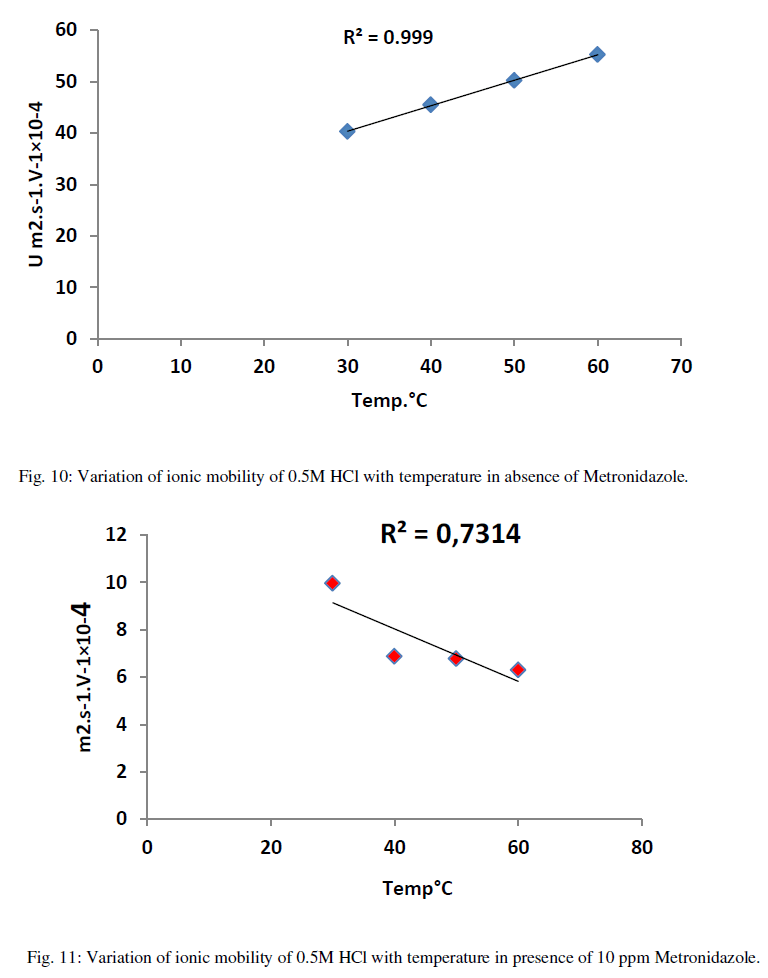
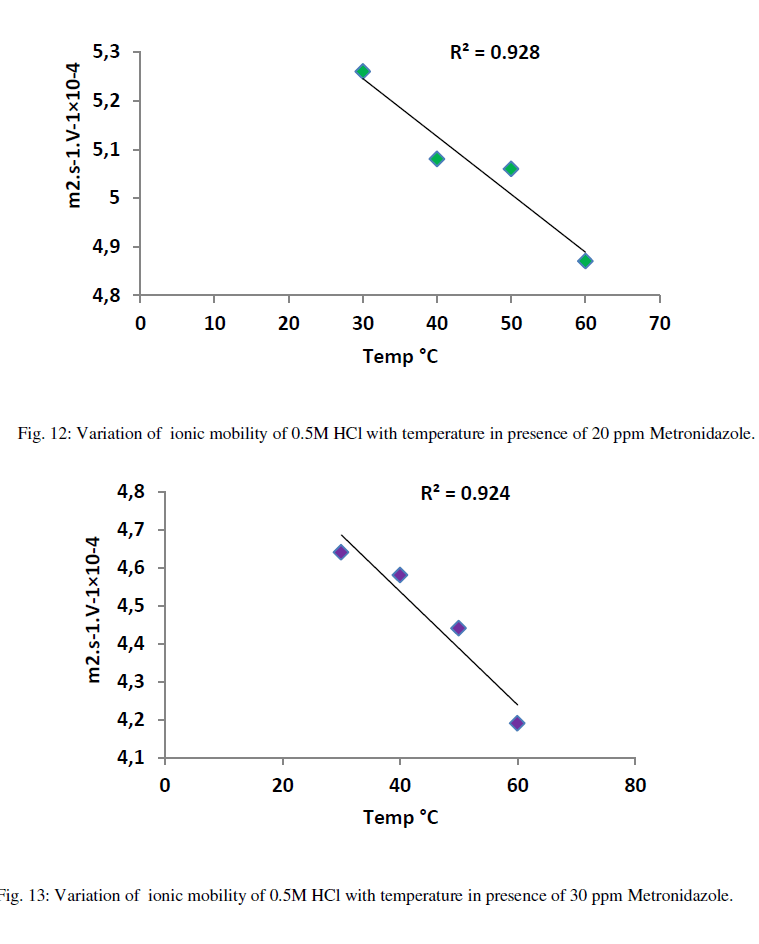
| As in Figure 8 above generally, the ionic mobility of hydrochloric acid is reduced as the concentration of Metronidazole is increased at different temperatures ranged (30-60) ºC especially at 60 ºC this can be attributed to reducing the activation energy of Metronidazole to inhibit the mobility of hydrogen and chloride ions toward the oppose electrodes thus, the reducing in molar conductance for hydrochloric acid as increasing the concentration of Metronidazole will be occurred at the above range of temperatures corresponding to the case of ionic mobility as shown in Figure 9 below: |
 |
| 2. Study the effect of temperature on the molar conductance of 0.50M HCl at constant concentration of Metronidazole: |
| The effect of temperature on the molar conductance for 0.5M HCl in the presence and absence of Metronidazole was studied which can be summarized in Tables (6-11) as below: |
 |
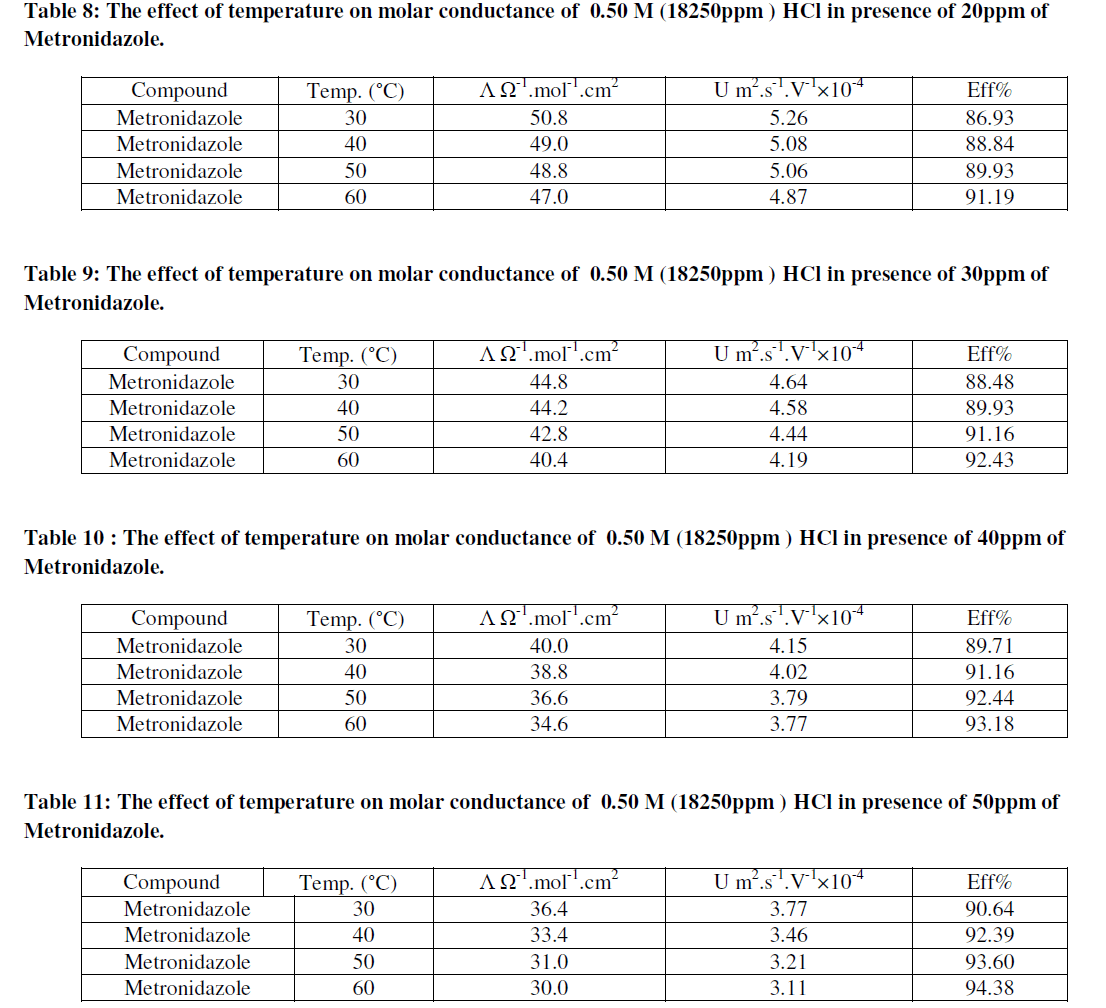 |
| As shown from the above tables the molar conductance of acid is increased as temperature increased due to the ionic mobility for acid ions is increased as in Table 6 but, when a Metronidazole is added at different concentrations i.e.,(10-50)ppm Tables (7-11), each one of the mentioned concentrations reduced the molar conductance of acid because , the ionic mobility of acid is reduced in presence of it where the efficiency of inhibition of conductivity is increased. Furthermore, the highest concentration of Metronidazole has he higher ability to reduce the molar conductance of acid compared with lowest concentration as in Tables (7-11). The relation between ionic mobility of acid and temperatures can be shown as in Figures (10-15) below: |
 |
 |
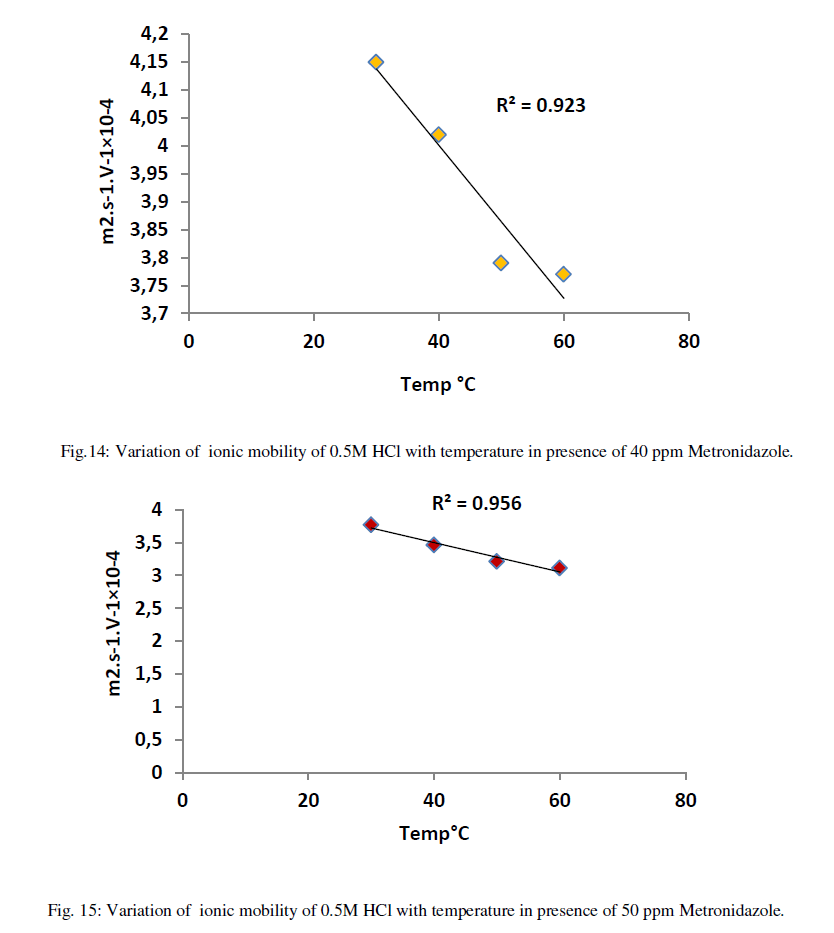 |
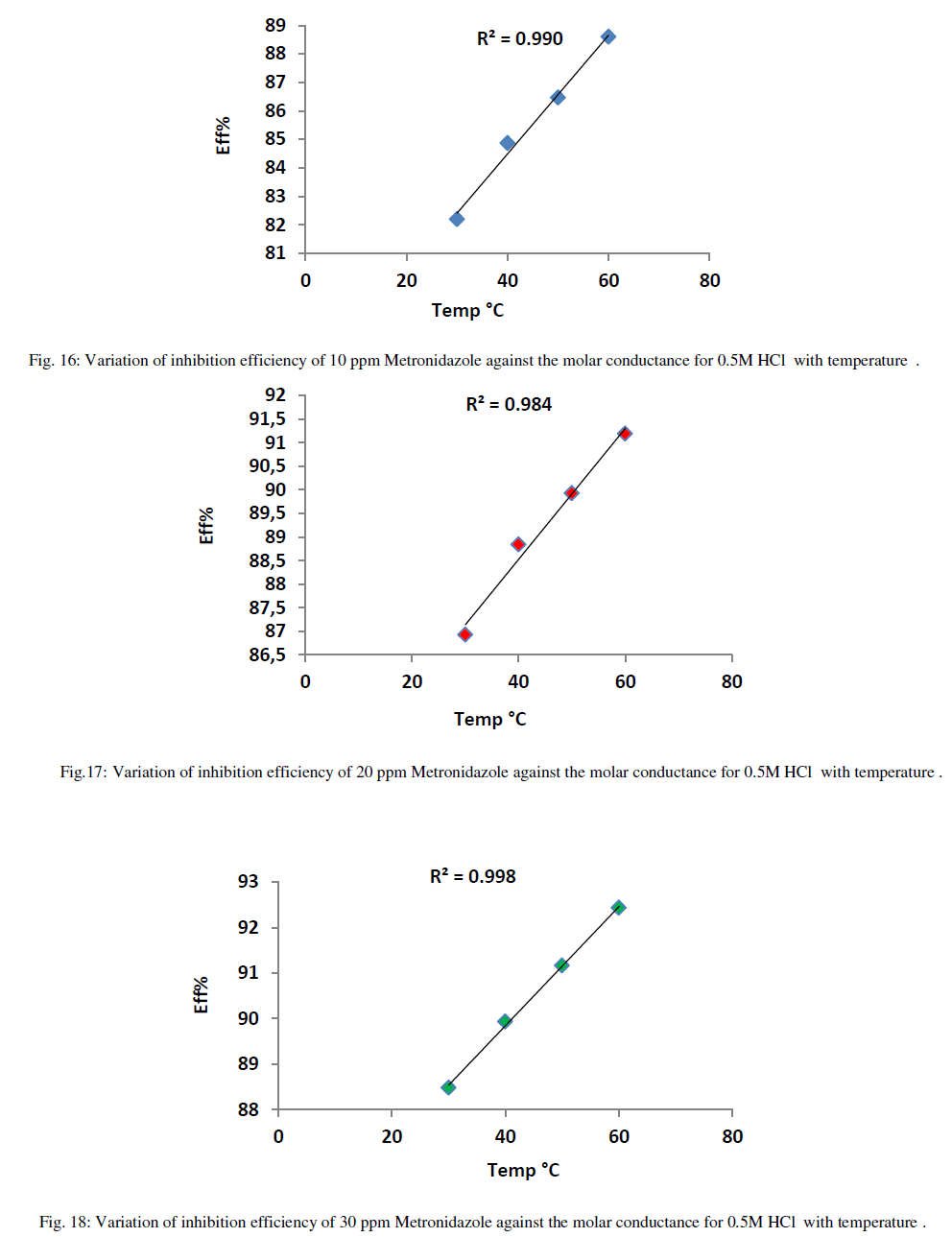
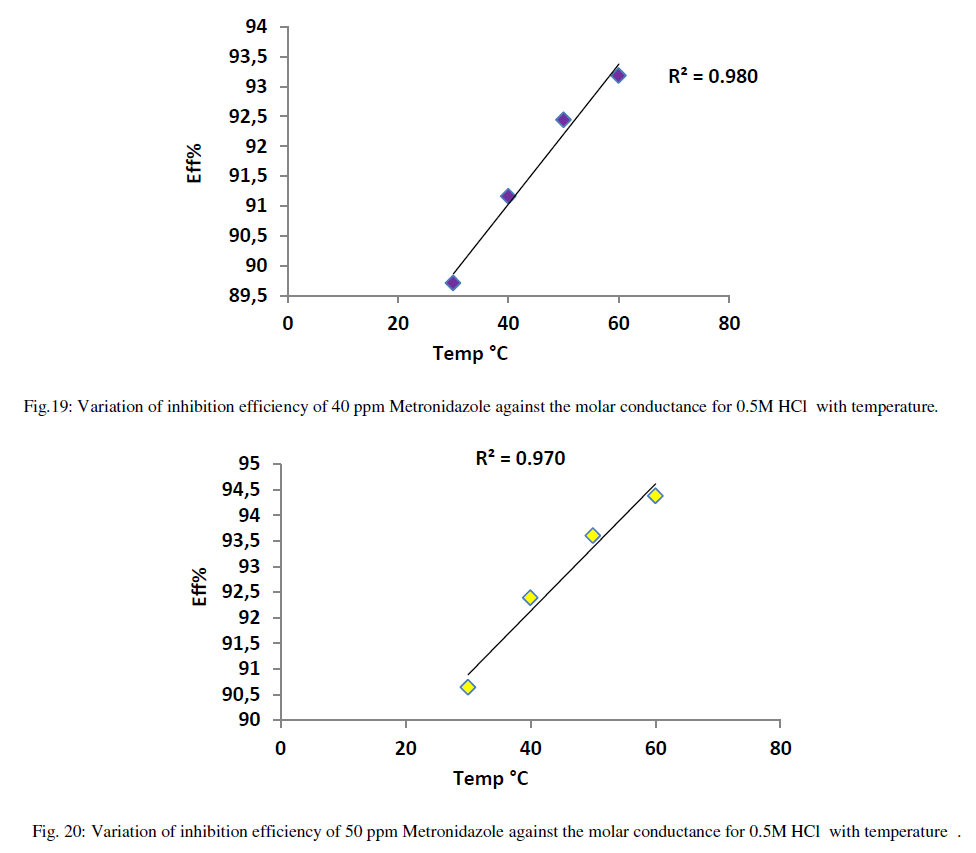
| On the other hand, the increasing of inhibition efficiency with temperatures is predominant at all concentrations of Metronidazole especially at higher concentration i.e., decreasing the activation energy that needed for Metronidazole as temperature increase to reduce the molar conductance of acid hence, increasing the concentration of Metronidazole make it has more strong ability to reduce the conductance of acid in other words increasing temperature leads increase the activation energy for acid ions to transport toward the oppose electrode in the conductance cell. The relation between inhibition efficiency and temperature is shown in Figures (16-20) below: |
 |
 |
IV. KINETIC STUDY |
| The molar conductance for hydrochloric acid in presence and absence of Metronidazole can be studied kinetically at temperatures ranged (30-60) ºC where, activation energy, enthalpy of activation, free energy of activation and entropy of activation are functions will be studied according to the particular equations i.e., Arrhenius equation is used to calculate the activation energy for hydrochloric acid in presence and absence of Metronidazole where the molar conductance of the acid can be considered as a function of activation energy[24]: |
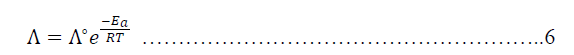 |
| Where Λ ia he molar conductance, Λº is the infinite molar conductance as Arrhenius constant, Ea is an activation energy in kJ.mol-1, R is Molar gas constant (8.314j.k-1.mol-1) and T is the absolute Temperature in k. Thus, Arrhenius equation is convert into natural logarithm for as below: |
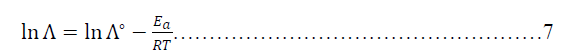 |
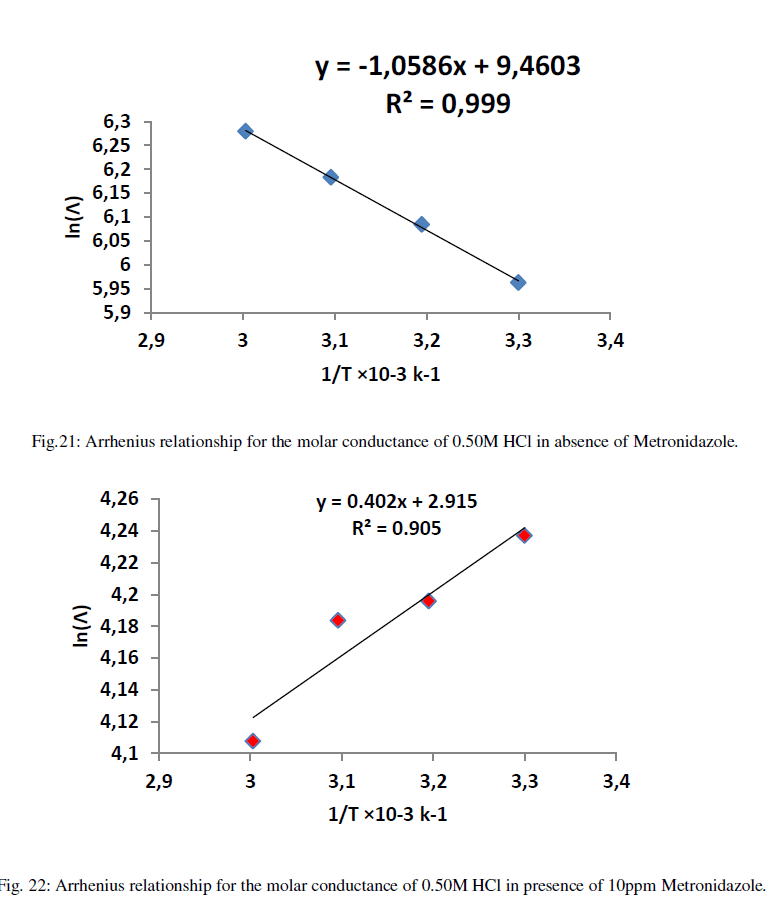
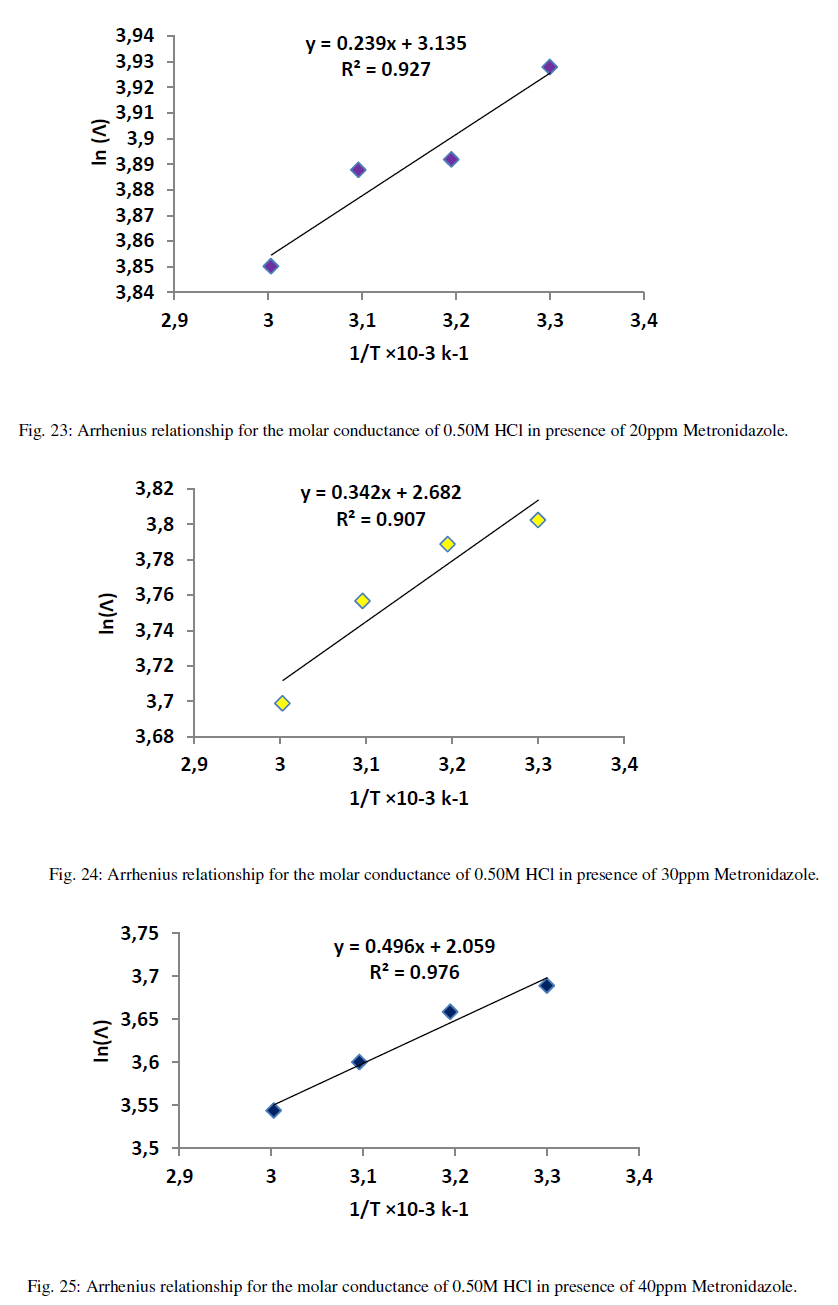
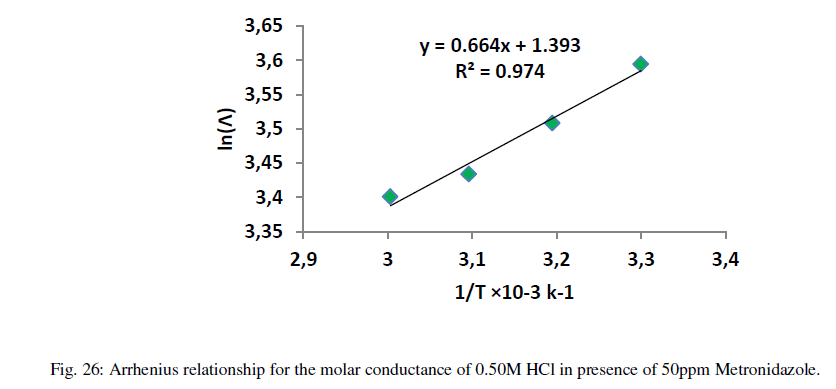
| When the relation between ln(Λ) against inverse of absolute temperature, the yielded slope is an activation energy and the intercept is the Arrhenius constant. . Figures (21-26) explain the relation between ln Ãâ°Ã⦠and the inverse of absolute temperature (Arrhenius equation) for 0.50M HCl in presence and absence of different concentrations from the Metronidazole respectively: |
 |
 |
 |
| Thus, enthalpy of activation is calculated according to the following equation which superimposed to Eyrlying equation: |
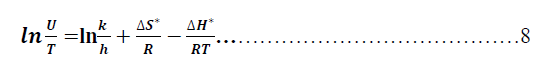 |
| Where ΔH* and ΔS* are the enthalpy and entropy of activation in kJ/mol and J.k-1.mol-1 respectively, k is a Boltzmann constant and U is the ionic mobility of hydrochloric acid. Furthermore, the free energy of activation ΔG* is calculated according to the following equation: |
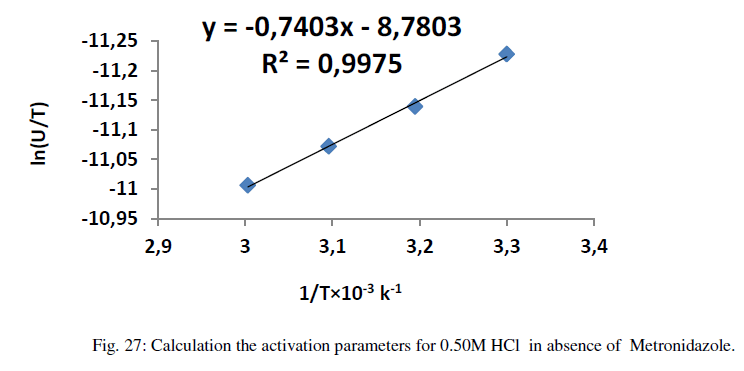
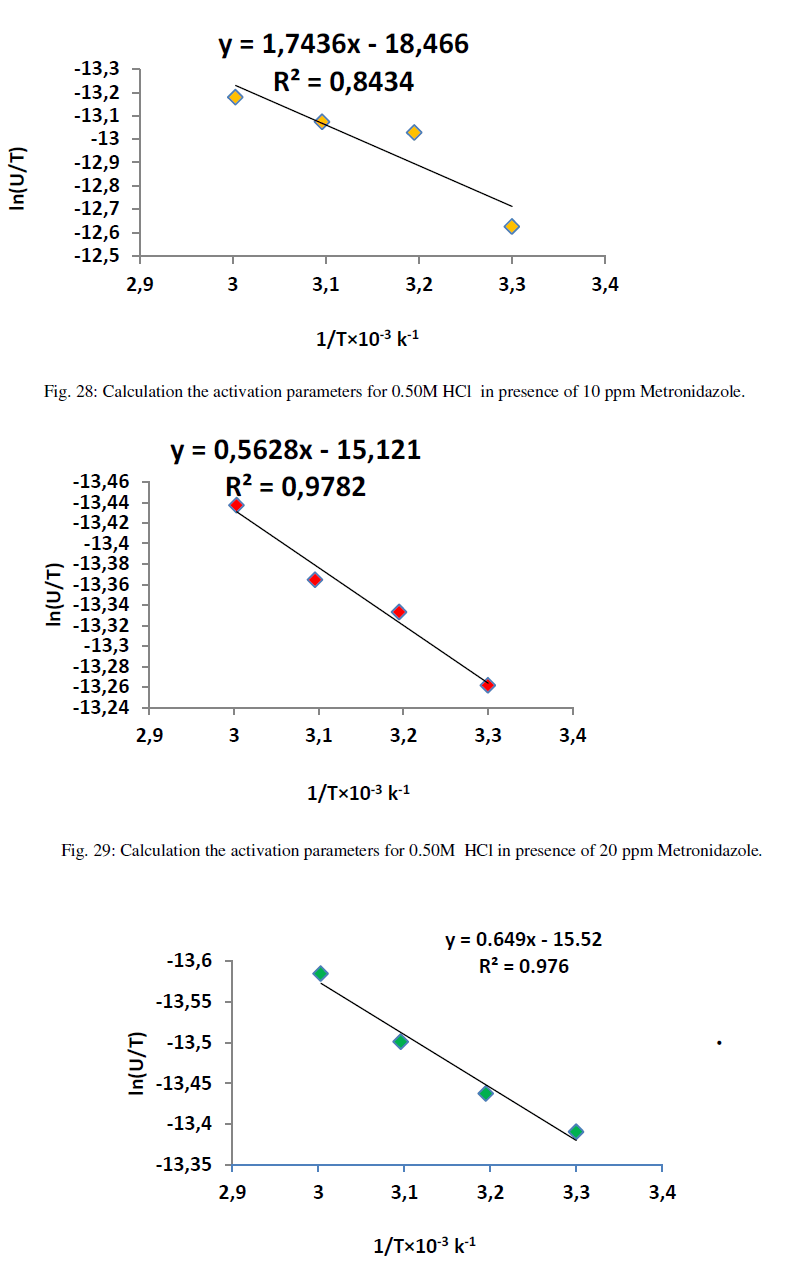
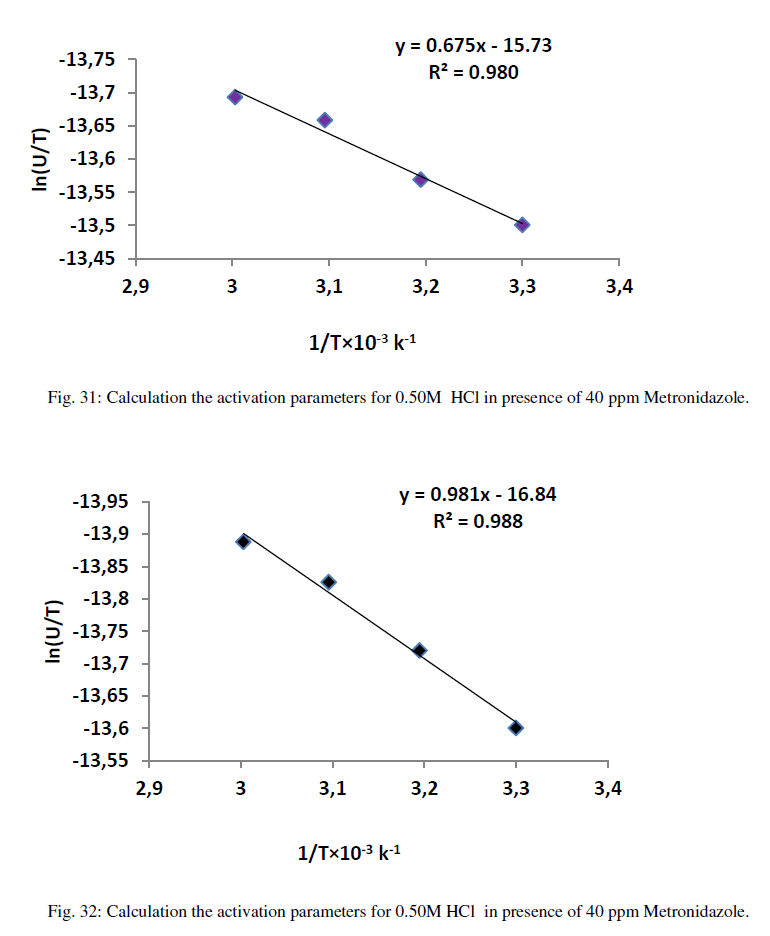
| Where ΔG* is the free energy of activation in kJ/mol. The calculation of enthalpy, entropy and free energy of activation can be explained in Figures (27-32) as below : |
 |
 |
| Fig. 30: Calculation the activation parameters for 0.50M HCl in presence of 30 ppm Metronidazole |
 |
| The kinetic activation functions is summarized in Table 12: |
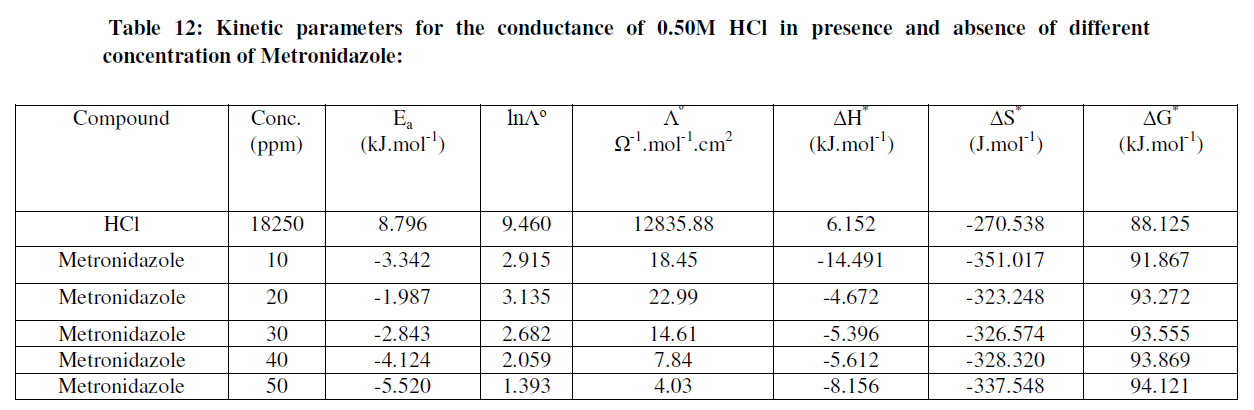 |
| Table 12 reveal that all functions of activation like energy of activation. Enthalpy of activation and energy of activation are positive i.e., endothermic and non-spontaneous process that corresponding with the highly value of infinite molar conductance (Ãâ°Ã⦰) in the mentioned table. When the Metronidazole is present, the activation energy will become negative that means the molar conductance process is reduced in presence of Metronidazole and this process is reduced as the concentration of Metronidazole is increased which can be indicated by reducing in (Ãâ°Ã⦰) values i.e., the molar conductance of acid decreases with increase in temperature in presence of all concentrations for Metronidazole this corresponding to negative values for enthalpy of activation in that can be attributed to the heat of adsorption for Metronidazole becomes larger than the activation energy of the intrinsic kinetic for the molar conductance for the hydrochloric acid i.e., The negative apparent electrochemical enthalpy of activation can be explained by a sufficiently negative enthalpy for the preceding adsorption equilibrium, which can lead to a negative apparent electrochemical enthalpy of activation for the overall process[24]. On the other hand, the free energy of activation for the molar conductance of hydrochloric acid in presence of Metronidazole will become more non-spontaneous which meant reducing the molar conductance in presence of . On the other hand, the negative values of entropy of activation is increased as concentration of Metronidazole increased insist that the mobility of both chloride and hydrogen ions toward the oppose electrodes in conduction cell is retarded by the Metronidazole [25]. |
V. THERMODYNAMIC STUDY |
| In this paragraph, thermodynamic functions like enthalpy of adsorption ΔHads, free energy of adsorption ΔGads and entropy of Adsorption ΔSads will be calculated related to the isotherms of adsorption as below: |
| Basic information on the interaction between the inhibitor and the alloy surface can be provided by the adsorption isotherm. In order to obtain the isotherm, the fractional coverage values θ as a function of inhibitor concentration must be obtained. It well known that θ can be obtained from the corrosion current via [26]: |
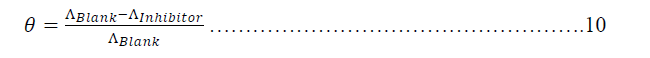 |
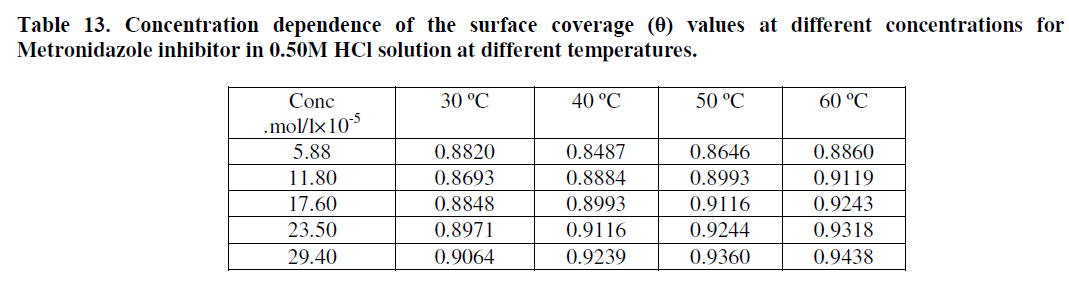
| Where θ is the value of retardation of hydrogen and chloride ions in hydrochloric acid to mobile toward the oppose electrode by Metronidazole. The θ values obtained in this way are shown in Table 13. |
| Attempts were made to fit these θ values to various isotherms including Frumkin, Langmuir,Temkin, and Freundlisch. Many adsorption isotherms were plotted for the Metronidazole and the best isotherm for each one of them was selected according to the R2 that has value near to unity value, According to θ is related to equilibrium adsorption constant(K and concentration of inhibitor morality C via |
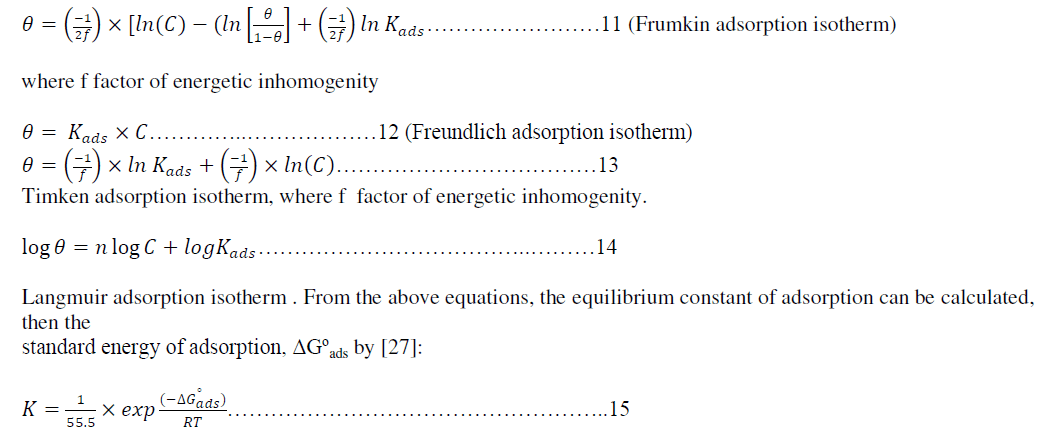 |
| where the value 55.5 is the concentration of water in the solution in mol.dm-3, K is the equilibrium constant of adsorption, C is the concentration in molarity. |
 |
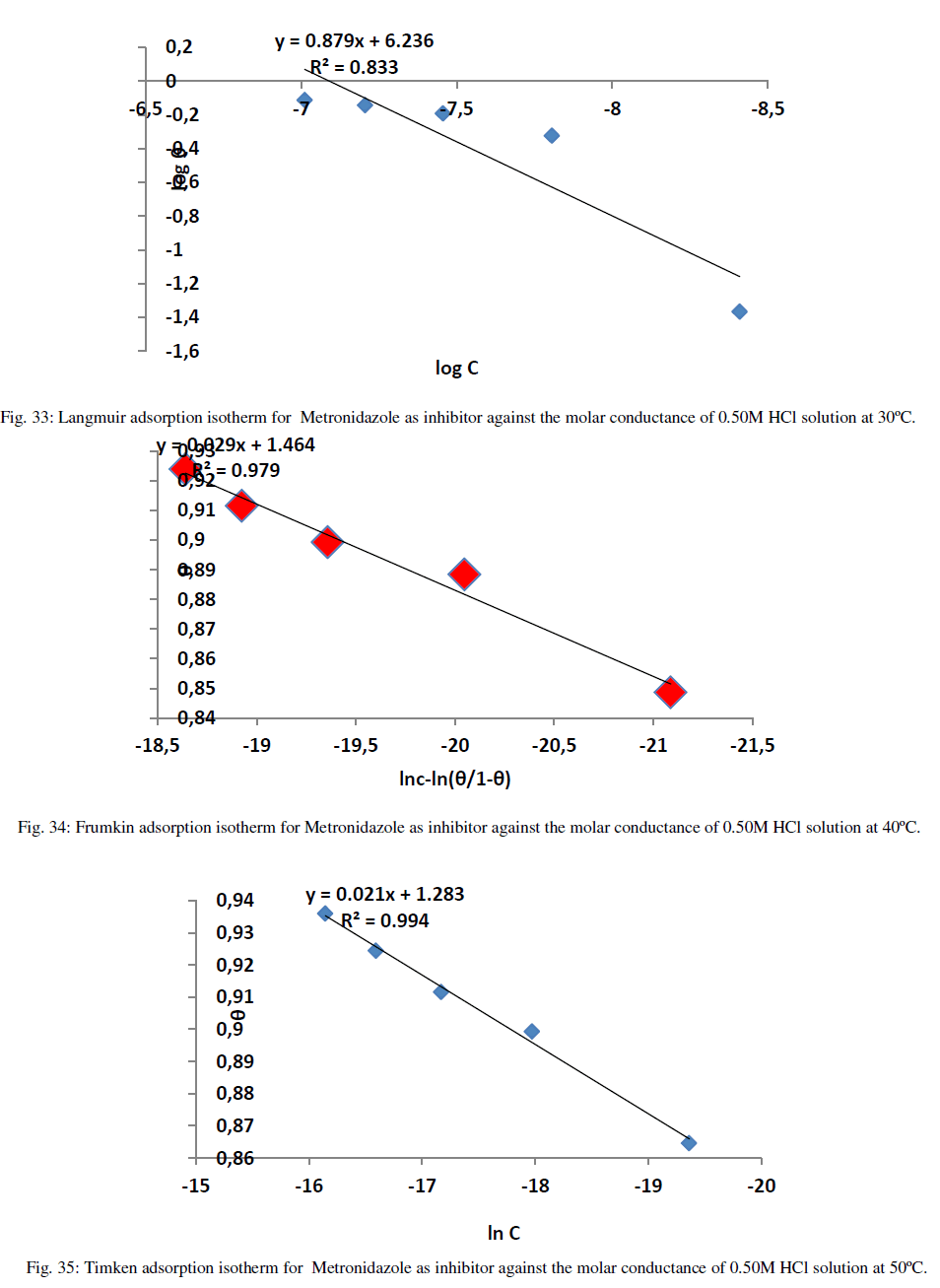
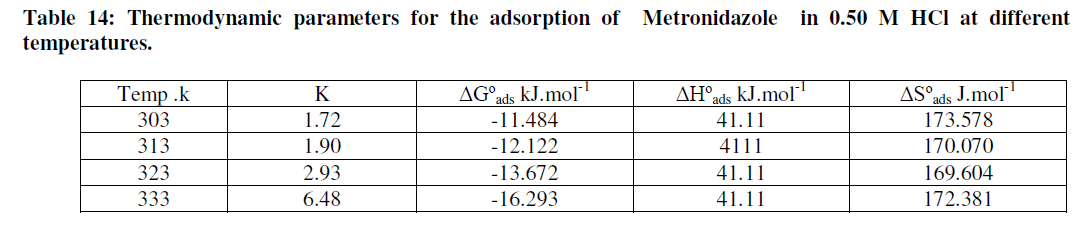
| The adsorption isotherms can be shown in Figures (33-36) below: |
 |
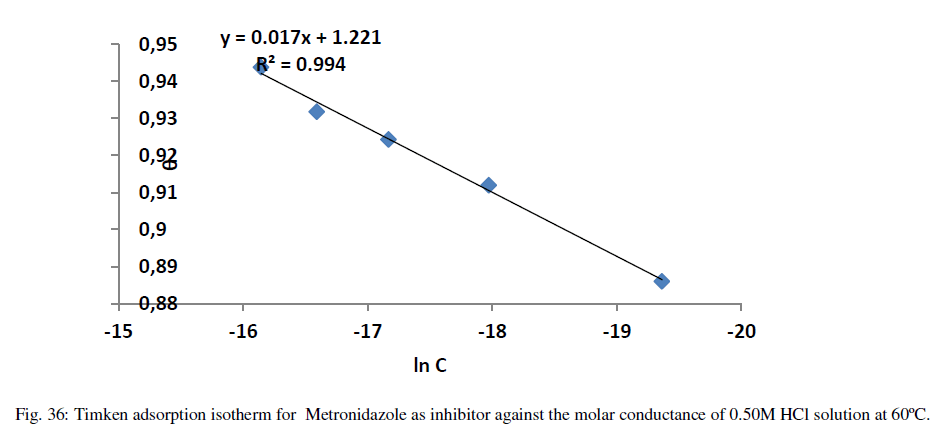 |
| Figures (33-36) show the type of adsorption isotherm for Metronidazole as inhibitor in 0.50M HCl at temperature ranged (30-60) ºC. The data are fitted to the Frumkin, Freundlisch Timken and Langmuir adsorption isotherms by using regression methods then K and ΔGºads can be obtained. In fact, at 30 ºC Metronidazole obey Langmuir Isother of adsorption while, at 40 ºC, the inhibitor (Metronidazole) obey to Frumkin adsorption isotherm but, both of 50 ºC and 60 ºC temperatures the inhibitor obey to Temkin adsorption isotherm as shown above The correlation coefficient (R2) was used to choose the isotherm that best fit experimental data for each one of the temperatures in this study[28]. Hence, the equilibrium constant, free energy of adsorption ΔGºads , enthalpy of adsorption ΔHºads and entropy of adsorption ΔSºads where, the following equation can be used to calculate a thermodynamic functions: |
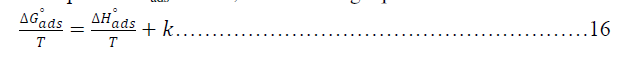 |
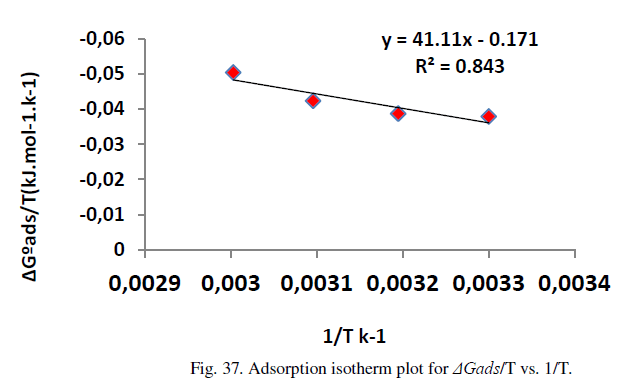
| The variation of ΔGºads /T with 1/T gives a straight line with a slope that equals ΔHºads (Figure 37) . It can be viewed from the Figure that ΔGºads /T decreases with 1/T in a linear manner. The calculated values are shown in Table 14. The adsorption heat could be approximately regarded as the standard adsorption heat under experimental conditions. The positive sign of ΔHºads in HCl solution indicates that the adsorption of inhibitor molecule is an endothermic process[27]. |
 |
| On the other hand, enthalpy of adsorption is calculated according to equation 16 as shown in Figure 37 below: |
 |
 |
| The negative values of free energy of adsorption (ΔGº ads) indicate that the adsorption process is spontaneous and the adsorbed layer on Metronidazole surface is stable [29] . On the other hand, endothermic adsorption reaction data are obtained in addition to ΔSº ads values in the presence of Metronidazole are positive, meaning a disordering in presence of inhibitor is increased in order to the inhibitor is adsorbed on the metal surface. On the other hand, when the data in Table 14 is compared with the inhibition efficiency in Tables 6-11 indicate that increasing in efficiency as temperature increased which is attributed to chemical adsorption mode, thus , at high degree of coverage, the diffusion through the surface layer containing the inhibitor and corrosion products become the rate determining step of the metal dissolution process i.e., the adsorption mode for Metronidazole is chemisorptions mode[22]. |
VI. CONCLUSION |
| There are several conclusions can be summarized as below: |
| 1. Metronidazole acts as a good retarder for the molar conductance of 0.50M HCl at different concentration i.e., (10- 50) ppm at constant temperature where as the concentration of Metronidazole increased , the molar conductance of acid increased at all temperatures tin this study. |
| 2. The molar conductance of acid is reduced as temperature increased in range (30-60) °C reducing the molar conductance is 94.38% which encourage us to use the Metronidazole as corrosion inhibitor for the metals or alloys in acidic media. |
| 3. It is noticed that the activation energy for the molar conductance of acid become a negative in presence of Metronidazole compared with its value in absence of it (positive) which meant the Metronidazole has the strong ability to inhibit the conductance of acid which correspond with the negative values for the enthalpy of activation for the molar conductance of acid due to the heat or enthalpy of adsorption process of HCl on the paracetamol is greater than the activation process for the molar conductance of acid. |
| 4. The free energy of activation for the molar conductance of acid is positive whether in presence or absence of Metronidazole but, the non-spontaneous property is increased in presence of paracetamol at all its concentrations. |
| 5. The entropy of activation for the molar conductance of acid is negative whether in presence or absence of Metronidazole but it increased as the Metronidazole is present which meant that an order for the mobility of both hydrogen and chloride ions are taken placed i.e., the mobility of both ions are restricted. |
| 6. The adsorption of Metronidazole is obey to different adsorption isotherm which meant that the temperature is affected on the behavior of the Metronidazole at ranged (30-60) °C where, the equilibrium constant of adsorption is increased as temperature increased, the adsorption process become more spontaneous and an endothermic adsorption process which accompanied by the positive entropy values, which meant a chemical adsorption mode is occurred for Metronidazole. |
References |
|