ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Zafar A. Siddiqi1, Istikhar A. Ansari2, Farasha Sama2, M. Shahid4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Functionalized dicarboxylic acids are potential candidates to act as chelating ligands in coordination chemistry since they have their own special characteristics of bonding to transition metal ions. Pyridine-2,6- dicarboxylic acid (H2Pda) is such a planar and versatile ligand for synthesizing a number of mono- and poly nuclear transition metal complexes. The homo dinuclear complex, [Co2(Pda)2(H2O)5]·2H2O is prepared and characterized using spectral and single crystal X-ray diffraction techniques. Analytical and ESI mass data are consistent with the proposed molecular stoichiometry of the complex. FTIR data confirmed the binding mode of the ligands to Co(II) ion. Crystal data [i.e., a = 8.353(5)), b = 27.164(5), c = 9.600(5) (Å), α = 90.00, β = 98.08(5) and γ = 90.00°] show that the complex crystallize in monoclinic systems with the space groups P21/c. Cyclic voltagammogram of the complex ascertain the presence of CoII‒CoIII quasi-reversible redox couple in solution.
Keywords |
| Pyridine 2,6,dicarboxalic acid; homo-dinuclear complex; X-ray diffraction, FTIR. |
INTRODUCTION |
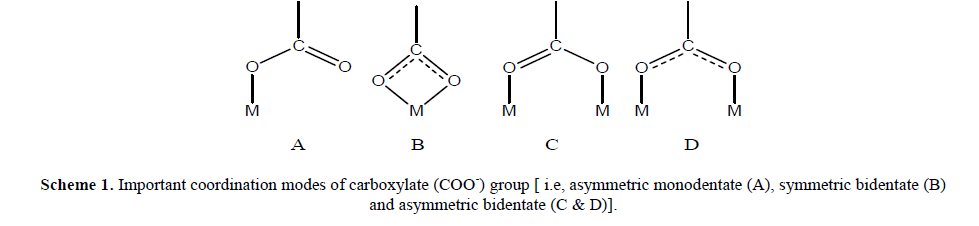
| The science of polynuclear complexes where metal ions are connected by bridging organic ligands has recently become a challenging issue for chemists, bio and nano technologists [1-3]. Coordination chemistry of functionalized carboxylate species has been an attractive subject from the bioinorganic point of view because the carboxylate group of glutamate and aspartate works as a supporting ligand for the metal centers in various metalloproteinase [4,5]. Recent investigations on metalloproteins have revealed that the carboxylate group plays an important role for structural holding and proton transfer via hydrogen bonding interactions in proteins. It exhibits a diversified coordination mode (Scheme 1) and provides an effective hydrogen bonding interaction with other protic group [6,7]. Bimetallic μ- carboxylate complex has been the most attractive synthetic target in view of the fact that the carboxylate-bridged bimetallic core is the common structural motif of the various O2- metabolic non-heme Fe- and Mn-proteins [8–10]. For example, all kinds of rigid pyridine dicarboxylic acids (2,3-, 2,4-, 2,5-, 2,6-, 3,4-& 3,5-) are good choices for constructing MOFs due to the coexistence of reliable pyridyl groups as well as carboxylate moieties with rich coordination modes [11–17]. With these ligands, a large number of beautiful networks of ingenious design have been constructed [18–23]. Metal complexes of pyridine carboxylic acid sand some of its derivatives have also been successfully used as model systems for the design of new metallo-pharmaceutical compounds [24]. The dicarboxylic acid analogue especially, pyridine-2,6-dicarboxylic acid also known as dipicolinic acid, is one of the most suitable ligand system for modeling potential pharmacologically active compounds because of the low toxicity, amphophilic nature and diverse biological activities [25-27]. This contemplated us to carry out synthesis and characterization of a bimetallic complex of H2Pda Co(II) ion. |
 |
II. EXPERIMENTAL |
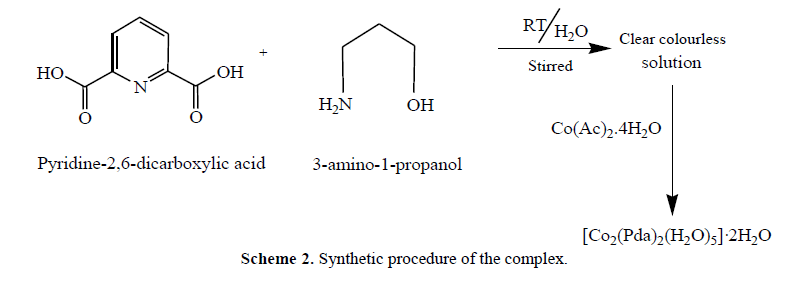
| Materials and methods All the solvents and the reagents were of AR grade and used as received. Metal salts (Merck), and pyridine- 2,6-dicarboxylic acid (Aldrich) were used as received for the syntheses. Synthesis of the complex [Co2(Pda)2(H2O)5]·2H2O Pyridine-2,6-dicarboxylic acid (0.34 g, 2.0 mmol) taken in 10 ml of ethanol was refluxed with 3-amino-1- propanol (0.30 ml, 4.0 mmol) for 6 h. An ethanolic solution of Co(Ac)2.4H2O (0.50 g, 2.0 mmol) was then added drop wise to the resulting solution. Stirring was continued for 10 h producing a purple color solid at room temperature. The solid was recrystallized in hot water yielding brown coloured crystals suitable for X-ray crystallography. Physical measurements FT-IR spectrum of the compound was recorded as KBr discs on Perkin Elmer Model spectrum GX spectrophotometer. Melting point was determined by open capillary method and are uncorrected. Thermal gravimetric analysis (TGA) data were recorded from room temperature up to 600 0C at a heating rate of 20 0C/min. The data were obtained using a Shimadzu TGA-50H instrument. Single crystal X-ray data of complex was collected at 100 K on a Bruker SMART APEX CCD diffractometer using graphite monochromated MoKïÃÂá radiation (ïÃÂì = 0.71073 Å) [28-33]. Cyclic voltammetry (CV) was performed on EG&G PAR 273 Potentiostat/Galvanostat equipped with an IBM PS2 computer with EG&G M270 software for data acquisition. The three-electrode cell configuration comprised of, a platinum sphere, a platinum plate and Ag(s)/AgNO3 were used as working, auxiliary and reference electrodes, respectively. |
III. RESULTS AND DISCUSSION |
| Complex was synthesized according to the procedure given in Scheme 2. The complex was fairly soluble in organic solvent and non electrolytic in nature (ïÃÂìm<50). The analytical data were consistent with the purposed stoichiometry of complex. The complex gave peaks at m/z 538 (48%) corresponding to the molecular ion fragments [Co2(Pda)2(H2O)5]+ in ESI Mass spectrum. A peak at m/z 226 (40%) is characteristic of [Co(Pda)]+ as the ultimate species in the ESI MS spectrum of the complex. |
 |
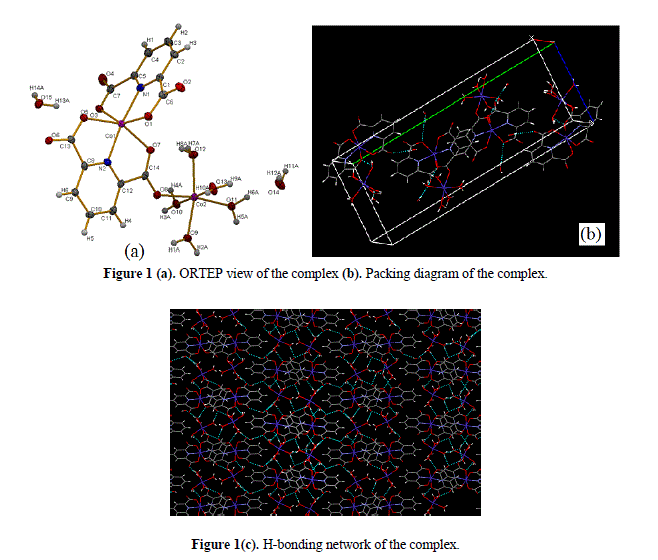
| FT-IR spectral studies The important frequencies observed in FT-IR spectrum of the compound with their assignments are given in Section 2. The νasym(COO) and νsym(COO) stretching vibrations characteristic of the coordinated carboxylate anion were indicated as strong intensity bands near 1632 and 1322 cm−1, respectively [34]. The observed large separation between νasym(COO) and ν sym(COO) stretching vibrations [i.e, Δν = νasym(COO–)–νsym(COO–) ~250] is characteristic [35,36] of an un-symmetrical monodentate mode of bonding of the corboxylate group in the present complex . The observed broad band in 3500–3200cm−1 region for the complex is characteristic of the presence of coordinated and lattice water molecules in the complex moiety. The ν(C=N) and ν(C=C) characteristic stretching vibration bands in the spectrum were indicated in the region 1580–1430cm−1. The carboxylate bridging (M–O–M) in the complex was ascertained by the appearance of medium intensity band at ~945cm−1 [34] in the spectrum of the molecule. Crystal structure of [Co2(Pda)2(H2O)5]·2H2O ORTEP view and packing diagram of the complex [Co2(Pda)2(H2O)5]·2H2O are shown in Figs.1(a) and 1(b), respectively. The structure clearly indicates that one Co(II) is surrounded by two Pda2- moiety and the other Co(II) is surrounded by five water molecules. Both the cobalt are joined together via a bridging corboxylate group of Pda2- moiety. The geometry of each Co(II) ion is distorted octahedral. Pda2- ligand binds the metal ion in di anionic tridentate [N,O,O] chelating mode. The dinuclear complex forms extensive H-bonding through lattice and as well as coordinated water molecules to give a supramolecular network [Fig. 1(c)]. |
 |
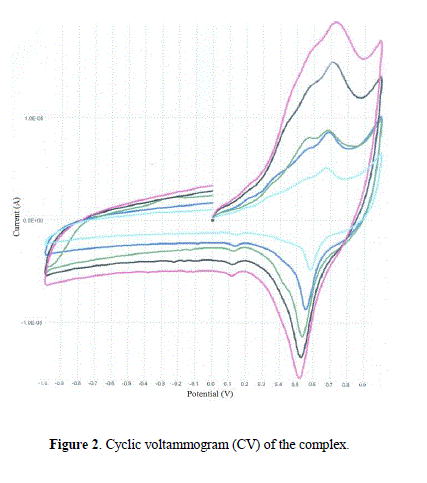
| Cyclic voltammetric (CV) studies The electrochemical redox properties of the complex have been studied using cyclic voltammetry in the potential range +1.0 to –1.0 V with reference to Ag/AgCl electrode at room temperature in the presence of tetrabutylammonium perchlorate as supporting electrolyte. Cyclic voltammogram of the complex is given as Fig. 2 at different scan rate (50, 100, 150, 200 and 250). The nature of peaks is almost similar at all scans rates indicating the stability of complex in solution. The cyclic voltammetric data show a cathodic peak, EP c =0.72 V coupled with an anodic peak, EP a =0.52 V, with E0 1/2=0.10 V. The peak current ratio (IP c/IP a ≠ 1) shows a quasi reversible redox couple in solution [37,38]. An additional irreversible peak at EP c = +0.53 V is consistence with an irreversible reaction in solution. The possible mechanism of change in oxidation state is as shown below: |
 |
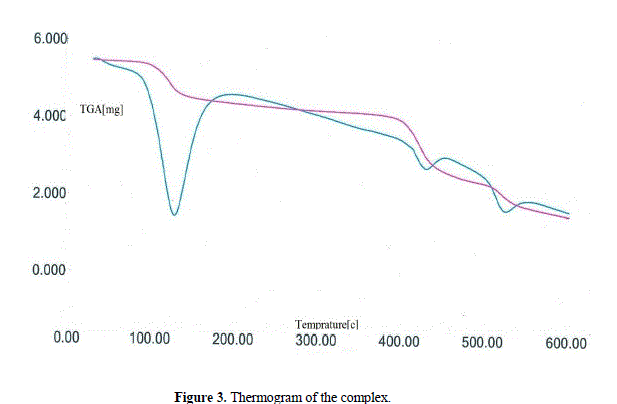
| Thermogravimetric analysis (TGA) In order to examine the thermal stability of complex, thermal analysis (TGA) was carried out in an N2 atmosphere at the rate of 5 °C per minute. The thermogram of compound shows a weight loss of ∼17.81% (expected = 17.89%) within the temperature range 80-150°C that corresponds to the release of all lattice water molecules [39,40]. On further increasing the temperature (~400 °C), again a weight loss of ∼5.31% was observed, consistent with the release of coordinated water molecules. Beyond this temperature (>400 °C), the compound starts to decompose (Fig. 3). |
 |
IV. CONCLUSION |
| A novel homo dinuclear complex with stoichiometry [Co2(Pda)2(H2O)5]·2H2O has been synthesized and characterized by spectroscopic and single crystal X-ray diffraction techniques. FTIR data suggested the presence of unusual coordination modes carboxylate moiety of the ligand. X-ray data ascertain a distorted octahedral geometry around each metal ion. The cyclic voltammetric studies confirm the presence of a quasi-reversible redox couple (CoII/CoIII) in solution. |
ACKNOWLEDGEMENTS |
| The authors thank Chairman, Department of Chemistry, AMU, Aligarh for providing necessary research facilities and UGC, New Delhi for financial assistance. |
References |
|