e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Pharmaceutical Chemistry, S.C.S College of Pharmacy, Harapanahalli. Karnataka, India
2Department of Pharmaceutical Chemistry, B.L.D.E.A’s College of Pharmacy, Bijapur, Karnataka, India
Received date: 12/03/2013; Revised date: 14/04/2013; Accepted date: 11/05/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
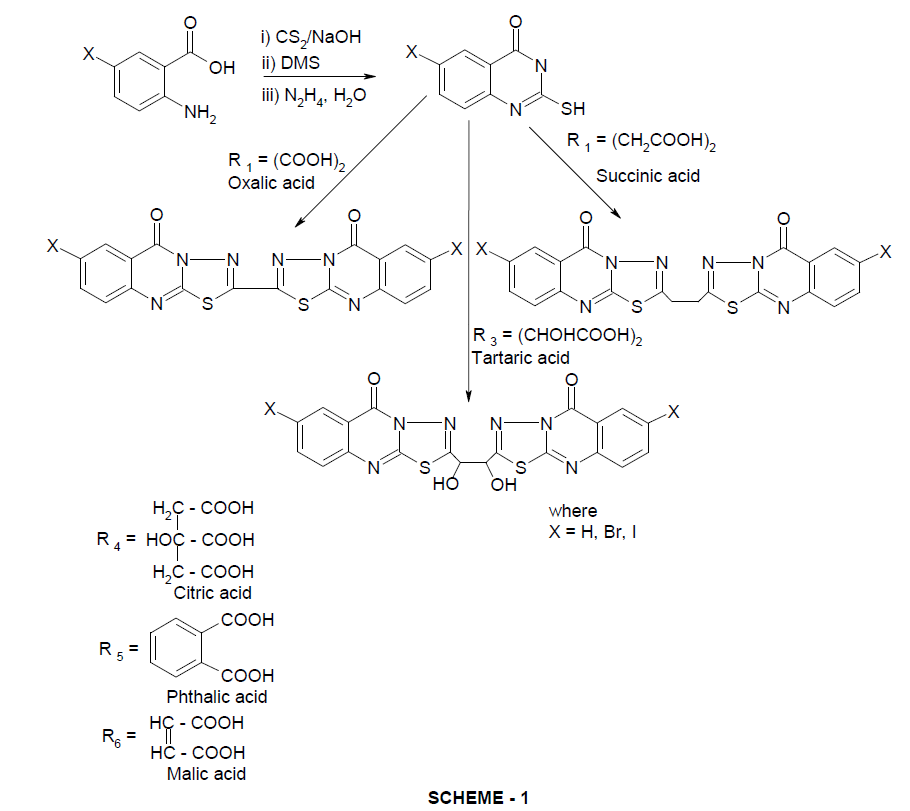
2.2'-bis-7-substituted-[1,3,4]thiadiazolo-[2,3-b] quinazolin-5-one (3,4,5) were synthesized by action of 3-amino-2-mercaptoquinazolinone-4(3H)one with various dicarboxilic acids in the presence of phosphorousoxy chloride. The newly synthesized compounds have been characterized by analytical and spectral (IR, 1HNMR and Mass) properties. Further, they have been screened for their antimicrobial, anti-inflammatory (in vitro and in vivo) and analgesic activity by standard method.
Fluorine, 1,3,4-Thiadiazolo, 3-amino-2-mercaptoquinazolinone-4(3H)one, Dicarboxilic acid
The rapid progress of organic Fluorine chemistry [1-5] since 1950 has been translated as a pathfinder to invent useful biodynamic agents in Medicinal and Biochemistry.The new generation antibiotics like Norfloxacin, Ciproflaxacin, Flufloxacin, Sporfloxacin and Ofloxacin which were incorporated with fluorobenzene moiety proved their efficacy as potent bio active molecules.
Having witnessed from literature, the enormous importance of quinazolinones [6-16] and their derivatives and in continuation of our investigations on these compounds, it is thought useful to synthesize and characterize some new quinazolinone fused heterocycles.
For this purpose 3-amino-2-mercapto-4-(3H)-quinazolinone and their nuclear substituted analogoues are selected as SYNTHONS. It is aimed to build-up some biologically and pharmacologicaly potent heterocycles while making use of amino and mercapto groups of the synthon. The present work is thus designed to achieve the synthesis of 2,2'-bis-7 substituted [1,3,4] thiodiozolo [2,3-b] quinazolin-5-one.
In view of the associated biological and pharmacological properties of these heterocyclics, it is planned to screen them for their possible activities, in conjunction with quinazolinones.
Melting point was determined by open capillary tube method and are uncorrected. T.L.C was run on silica gel G plates using chloroform : ethanol [9:1] as developing solvent for the purity of the compounds. I.R. Spectra were recorded on Shimadzu FTIR Spectrophotometer by using NUJOL MULL technique.
The compound 3-amino-2-mercaptoquinazolin-4(3H)-one (2) was synthesized by adding carbondisulphide (1.6 m1. 0.026 mol) and aqueous sodium hydroxide (1.2 m1, 20M) dropwise to a vigorously stirred solution of anthranilic acid (1, 0.02 mol) in dimethylsulfoxide (10 ml) at room temperature. After thirty min dimethylsulphate (2.5g. 0.02 mol) was added dropwisc under cooling with an ice bath. Stirring was continued for 3h, the reaction mixture was then poured into icc water and extracted with chloroform. The solvent was removed by distillation under reduced pressure. Thus the obtained methyl-N-(2-carboxyphenyl) dithiocarbamate was used for further reaction without purification. Hydrazine hydrate (8.6g. 0.2 mol. 80%) was added dropwise with stirring to methyl-N-(2-carhoxyphenyl) dithiocarhamate in cold condition. After completc addition, stirring was continued for 1½ hr at 500C and then it was poured into ice water, the solid obtained was filtered, washed with water dried and recrystallized from dimethylformamide-ethanol mixture to yield (2) as a white crystalline product.
A mixture of compound (2, 0.02 mole) oxalic acid/ succinic acid/ tartaric acid/ maleic acid/ citric acid/ phthalic acid/(0.01 mole) and POCL3 (15ml) was heated and reflux for 2-4 hrs while monitoring by TLC. Phosphorus oxychloride was removed from ensuring the completion of the reaction undr reduced pressure and cooled. The reduced pour on the crused ice (100g), while stirring the resultant solution was neutriilsed with sodium bicarbonate while cooling in ice bath. The precipitated soild was filteren, washed with cold water and dried. The crude product was recrystalise from dimethyl formamide.
Anti-microbial Activity [17-24]
The synthesized compounds are screened against bacterias like staphylococcus aureus (Gram +ve) and Escherichia coli (Gram -ve) and Bacillus subtillis (Gram +ve) and Pseudomonas aureus (Gram -ve) and fungi like Candida albicans and Aspergillus flavus to know their antimicrobial activity (by cup plate method).
Anti-inflammatory activity (in-vitro) [25,26]
The synthesized compounds are screened for anti-inflammatory activity by using inhibition of albumin denaturation technique which was studied according to Jayachandran E. and G.M. sreenivasa with slight modification.
The standard drug and test compounds were dissolved in minimum amount of dimethyl formamide (DMF) and diluted with phosphate buffer (0.2 M, pH 7.4). Final concentration of DMF in all solutions was less than 2.0%. Test solution (1 ml) containing different concentrations of drug was mixed with 1 ml of 1% mM Bovine albumin solution in phosphate buffer and incubated at 27°±1°C in incubator for 15 min. Denaturation was induced by keeping the reaction mixture at 60°±1°C in water bath for 10 min. After cooling the turbidity was measured at 660 nm (UV-Visible Spectrophotometer SL-159, Elico India Ltd.). Percentage of inhibition of denaturation was calculated from control where no drug was added. Each experiment was done in triplicate and average was taken. The Ibuprofen was used as standard drug.
Antibacterial activity by cup-plate method
Synthesized compounds have been evaluated for antibacterial activity at concentration of 50 μg/ml and 100 μg/ml by standard method, against following bacteria:
a) (i) Streptococci (ii) Staphylococus aureus gram (+ve).
b) (i) Pseudomonas, (ii) Escherichia coli gram (-ve).
All the compounds have been shown to exhibit a moderate broad spectrum of activity. The test compounds have been found to active against both gram (+ve) and gram (-ve) organisms.
The test compounds DK-2, DK-7, DK-8, DK-17 showed superior in it's antibacterial activity at lower (50μg/ml) and DK-2, DK-7, DK-11, DK-12, DK-15 at higher (100μg/ml) concentration against Streptococci gram (+ve), compare to standard drug procaine pencillin.
Compounds DK-3, DK-4, DK-6, DK-10, DK-16, DK-17 at (50μg/ml) and DK-2, DK-4, DK-7, DK-9, DK-12, DK-15 at (100μg/ml) showed promising antibacterial activity against Pseudomonas gram (-ve), bacteria compare to standard drug streptomycin.
Compounds DK-3, DK-7, DK-10, DK-12, DK-15 at (50μg/ml) and DK-1, DK-3, DK-7, DK-9, DK-12, DK-15 at (100μg/ml) showed moderate activity against Staphylococcus aureus gram (+ve) bacteria compare to standard drug procaine pencillin.
The compounds DK-4, DK-6, DK-8, DK-10, DK-13, DK-17 at (50μg/ml) and DK-2, DK-4, DK-8, DK-10, DK-13, DK-15, DK-17 (100 μg/ml) being same has been found exhibit relatively by more in their inhibitory action against Escherichia coli gram (-ve) bacteria, compare to standard drug streptomycin.
Antifungal activity
All the compounds were subjected to antifungal activity. For these activities Candida albicans and Aspergillus flavus fungal organisms were used. The Griseofulvin was used as standard.
Standard and synthesized compound were tested at two cone. Viz., 50μg/ml and 100 μg/ml. The compounds have shown activity, but none of them have shown better activity than standard.
Some of compounds like DK-1, DK-2, DK-4. DK-9, DK-11, DK-14 and DK-15 have shown activity almost equal to standard against Candida albicans at 100μg/ml cone. and DK-1, DK-8, DK-11 and DK 15 have shown activity near to standard at 50μg/ml conc. and the compounds DK-1, DK-2, DK-7 and DK-11, have shown activity near to standard against Aspergillus flavus organism at 100μg/ml cone. and DK-1, DK-2, DK-7, DK-8 and DK-11 at 100μg/ml conc. The remaining compounds have shown very low activity.
From this it is concluded that the synthesiled compounds have shown better activity agtainst Aspergillius flavus than Candida albicans.
Anti-inflammatory activity (in vitro model)
Synthesized compounds of 2,2'-bis-7 substituted-[1,3,4]-thiadiazolo-[2-3-b] quinazolin-5-ones have been evaluated for anti-inflammatory activity (in-vitro). Some of compounds promisingly inhibit albumin denaturation in comparison with standard drugs, ibuprofen exhibited 74.63% inhibition of albumn denaturation.
However eight out of eighteen compounds having more than 40% inhibition of albumin denaturation. Out of that DK-2, DK-3, DK-4, DK-6. DK-10, DK-12. DK13 and DK-14 showed 48.54%, 45.65%, 73.91%, 71%, 70.28%, 71.74%, 72.46% and 41.30% inhibition of albumin denaturation.
The authors are thankful to Shri. Sha. Bra. Chandramouleshwara Shivacharya Swamiji, President, Sri. T. M. Chandrashekaraiah M.A. Secretary, T.M.A.E. Society Harapanahalli. for providing necessary facilities through the Principal, S.C.S. college of Pharmacy, Harapanahalli to carry out this work.