ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Gurjaspreet Singh Department of Chemistry, Panjab University, Chandigarh, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
CH3(O)2SOSi(OC6H2Me2CH2)3N (1), CH3OSi(OC6H2Me2CH2)3N (2) and CH3(O)2SO.Si(OCH2CH2)3N (3) have been synthesized in high yield by the reaction of 1-isothiocyanato- silatranes with dimethyl sulfite and methyl methanesulfonate. These novel silatranes have been characterized by elemental analysis, infrared spectroscopy, 1H, 13C, 29Si NMR spectroscopy and mass spectrometry. Semi-empirical quantum mechanical calculations were done to study the N→Si transannular bond length.
KEY WORDS |
| Six membered silatrane, Methane sulfonato silatrane, Pentacoordinated silicon, Hypervalent compound, Five membered silatranes |
I. INTRODUCTION |
| Silatranes containing six-membered ring were synthesized as reported in literature [1-2]. It was shown that the silatrane systems with the larger rings allowed substituent effects associated with the axial position of the resulting trigonal bipyramidal structures (tbp) to be reflected in dramatically altering the Si-N bond interactions as well as in changes of the 29Si chemical shifts [3-7]. The use of dimethyl sulfite and methyl methanesulfonate as alkylating, alkoxylating and acetylating reagent in synthesis is well known in literature [8,9]. To my knowledge these are the first of type of silatrane containing1-methane sulfonato as exocyclic group to silicon in silatranes. In order to understand the reactivity of 1- Isothiocyanatosilatranes with dimethyl sulfite & methyl methane sulfonate, a detailed study of this reaction was undertaken. |
II. EXPERIMENTAL |
| All operations were carried out under dry nitrogen atmosphere. Solvents were freshly distilled under an inert atmosphere from sodium and sodium benzophenone (hexane), (tetrahydrofuran, diethylether) and phosphorus pentaoxide (acetonitrile and dichloromethane) before use. Bases such as triethanol amine (Aldrich) were refluxed over potassium hydroxide pellets and distilled under dry nitrogen atmosphere before use. Other starting materials such as silicon(IV) chloride (Aldrich AR grade), absolute alcohol (Bengal chemicals), 2,4-dimethyl phenol (E-Merck), hexamethylene tetramine (Aldrich) and potassium thiocyanate (E-Merck) were used as supplied. Tris-(2-hydroxy-3,5-dimethyl benzyl)amine [2], dimethyl sulfite [8], methyl methanesulfonate [9], triethoxyisothiocyanatosilane [7], 1-isothiocyanato six membered ring silatrane [1] and 1-isothiocyanato five membered ring silatrane [10] were synthesized according to procedure reported earlier. Solvents were purified according to standard procedures [11]. IR spectra were obtained as thin films or nujol mulls on Perkin-Elmer RX-1 FTIR spectrophotometer. 1H (300.4 MHz), 13C (75.45 MHz), 29Si NMR (59.60 MHz) spectra were obtained on JEOL AL 300 instrument. Chemical shifts were reported with respect to TMS as external standard. Mass spectral measurements (EI, 70ev) were carried out with VG Analytical (70-S) spectrometer. The elemental analysis were performed using PERKIN-ELMER (model 2400) C, H, N analyzer. The content of sulfur and silicon was determined by standard gravimetric methods. |
III. SYNTHESIS |
Reaction of 1-Isothiocyanato six-membered silatrane with dimethyl sulfite |
| The reaction of equimolar quantities of 1-isothiocyanato six-membered ring silatrane (1.67 g 3.33 mmol) and dimethyl sulfite (4.00 ml 47.00 mmol) has been carried out at 110 ïÃâðC by refluxing for 4 h. Dimethyl sulfite was taken slightly in excess. The solid was filtered under the dry nitrogen atmosphere, washed with hexane (2 mL), and dried under vacuum to give a mixture of compounds 1 and 2. Yield: 86%. M.P: 211-218 οC Anal. calcd. for C56H66O10N2Si2S(Mol mass 1014): C:66.27; H:6.51; N:2.76; S:3.15; Si:5.52%. Found C, 66.01; H, 6.59; N, 2.71; S, 3.09; Si, 5.50%. IR (Nujol, KBr, cm-1): ïÃÂõ = 1070 [Si-O(S)], 1145 (Si-OCH3), 1020, 1040, 1145 (SO3), 958, 915, 677 (skeleton mode of silatrane). 1H NMR (CDCl3): δ = 2.17, 2.19 (s, 9H, aryl-Me), 2.20, 2.24 (s, 9H, aryl Me), 4.21, 4.25 (s, 6H, NCH2), 6.89, 6.90 (s, 3H, Aryl), 6.91, 6.93 (s, 3H, Aryl), 3.42 (s, 3H, OCH3), 2.78 (s, 3H, SCH3). 13C NMR (CDCl3): δ = 16.11, 16.13 (Aryl-CH3), 19.21, 19.23 (Aryl- CH3), 54.91, 54.95 (NCH2), 49.91 (OCH3), 39.24 (SCH3), 116.47-150.96 (Aryl carbons). 29Si NMR (CDCl3): δ = -123.13 (1-methoxy silatrane); δ = –129.27 (1-methanesulfonato silatrane). |
Reaction of 1-Isothiocyanato six-membered silatrane with methyl methanesulfite |
| In a round-bottomed flask a solution of 1-isothiocyanato six membered ring silatrane (0.69 g, 1.37 mmol ) and methyl methanesulfite (4.00 ml, 47.00 mmol) were stirred for 4 h at 125 ïÃâðC. The contents were allowed to retain room temperature. To this viscous liquid anhydrous hexane was added, when a white solid was formed. The solid was filtered under the dry nitrogen atmosphere, washed with hexane (2 mL), and dried under vacuum to give 1. Yield: 94%. M.P: 215 οC. Anal. calcd. for C28H33NO6.SiS (Mol mass 539): C: 62.33; H: 6.12; N: 2.60; S, 5.94; Si, 5.20%. Found: C: 62.29; H: 6.09; N: 2.57; S: 5.96; Si: 5.18%. IR (Nujol, KBr, cm-1): ïÃÂõ = 1070 (Si-OCS), 1022, 1108 (SO3) 960, 925 and 635(skeleton mode). 1H NMR (CDCl3) δ = 2.13, 2.30(s, 9H, Ar-CH3), 4.07 (s, 6H, NCH2), 6.67, 6.86 (s, 3H, Ar-H), 2.94 OS(O)2CH3. 13C NMR (CDCl3) δ = 16.14, 20.23 (Ar-CH3), 56.80 (NCH2), 41.23 OS(O2CH3), 116.76, 126.10, 128.73, 129.12, 133.80 and 151.85 (aromatic carbons). 29Si NMR (CDCl3): δ = –115.67. |
Reaction of 1-Isothiocyanato five-membered silatrane with methyl methanesulfite |
| In a round-bottomed flask a solution of 1-isothiocyanato five membered ring silatrane (0.50 g, 1.56 mmol) and methyl methanesulfite (4.00 ml, 47.0 mmol) was stirred for 4 h at 125 ïÃâðC. The contents were allowed to retain room temperature and diethyl ether (2 mL) was added, and a white solid formed. The solid was filtered under the dry nitrogen atmosphere, washed with diethyl ether (2 mL), and dried under vacuum to give 3. Yield: 91%. M.P.: 209 οC. Anal. calcd. for C7H15O6NSiS (mol mass 269): C: 31.23; H: 5.58; N: 5.21; S: 11.90; Si: 10.41%. Found: C: 31.23; H: 5.62; N: 5.19; S: 11.46; Si: 10.39%. IR (Nujol, KBr, cm-1): ïÃÂõ = 1020, 1040, 1145 (SO3), 1070 (Si-OCS) 940, 910, 617 (skeleton mode). 1H NMR (CDCl3): δ = 3.95 (s, 6H, OCH2), 2.91 (s, 6H, NCH2) 2.81 (s, 3H, SCH3). 13C NMR (CDCl3): δ =57.85 ppm (OCH3), 50.19 (NCH2), 39.21 (SCH3). 29Si NMR (CDCl3): δ = –93.71. |
IV. RESULTS AND DISCUSSION |
| The use of dimethyl sulfite as alkylating, alkoxylating and acetylating reagent in organic synthesis is well known in literature [8,9]. However, this reagent has not been fully exploited in the area of main group element chemistry. |
| Reaction of 1-isothiocyanato six membered silatrane with dimethyl sulphite in excess at 125 ïÃâðC for 3 h results in a clear solution. Subsequent heating of reaction mixture for 3 h followed by the addition of dry diethyl ether yields a white solid. This white solid was filtered & washed with diethyl ether in the dry nitrogen atmosphere and dried under vacuum. Elemental analyses of white solid confirmed the formation of 1-methanesulfonato silatrane 1 and 1-methoxy silatrane 2 in equimolar mixture. The reaction may be represented as below (Scheme 1): |
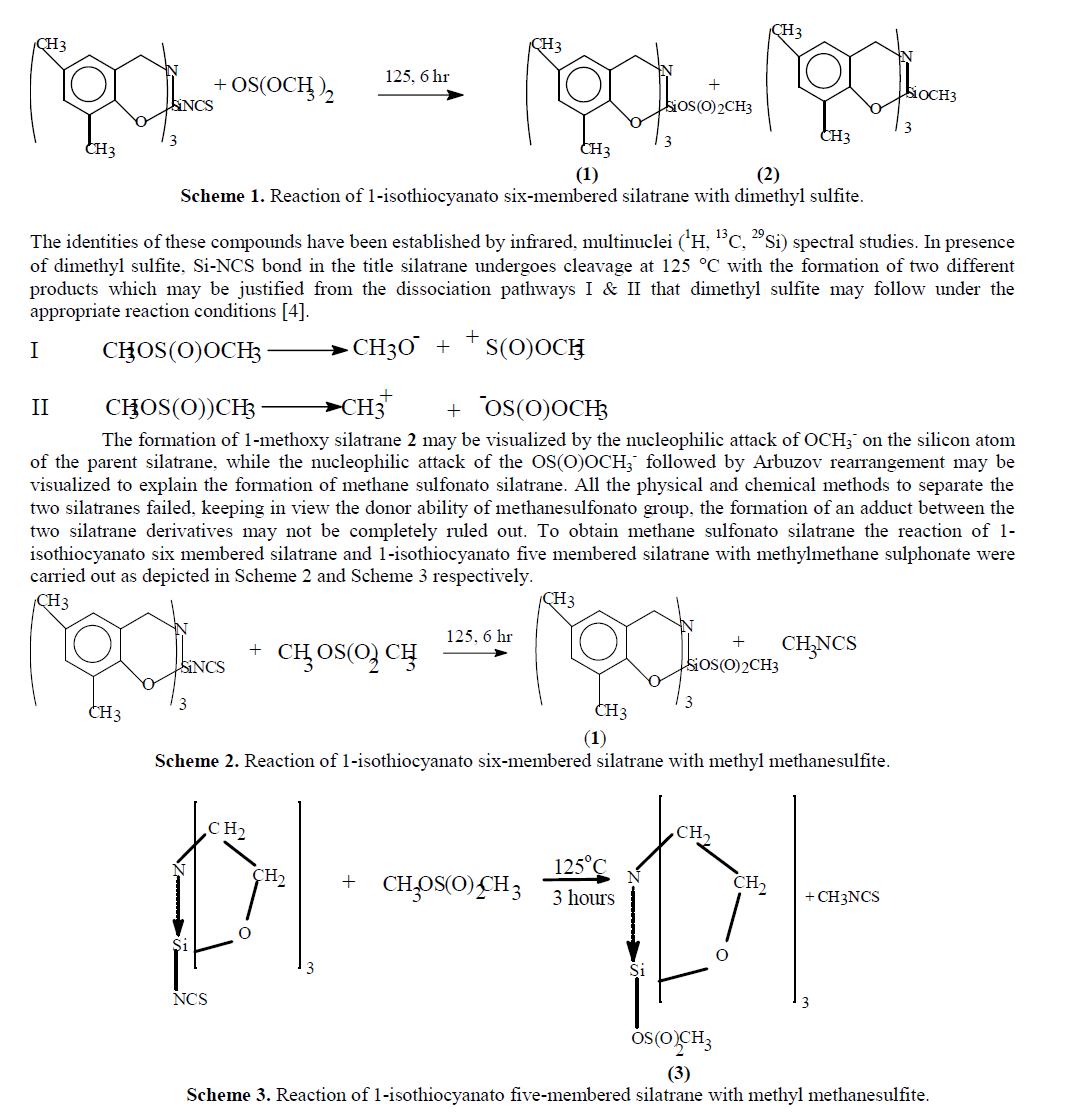 |
| To supplement our experimental work, we have also performed some semi-empirical quantum mechanical computations using AM1, PM3, MNDO & MNDO/d methods (Table 1 & 2). |
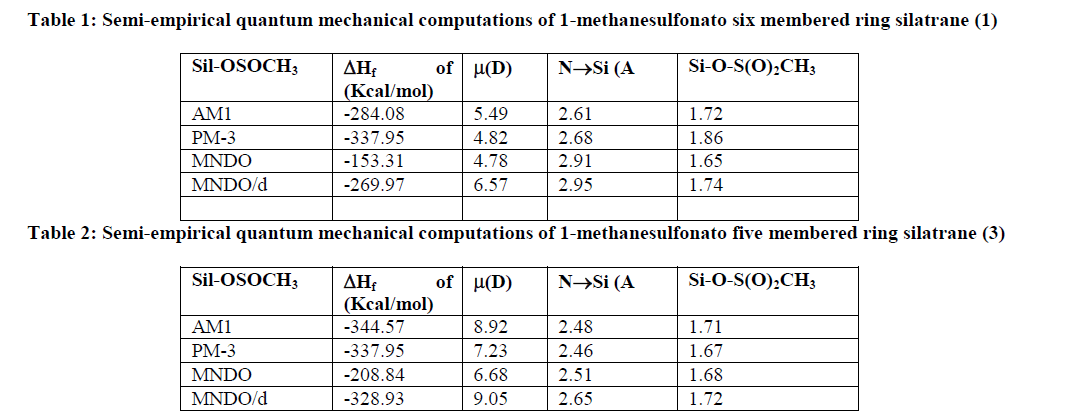 |
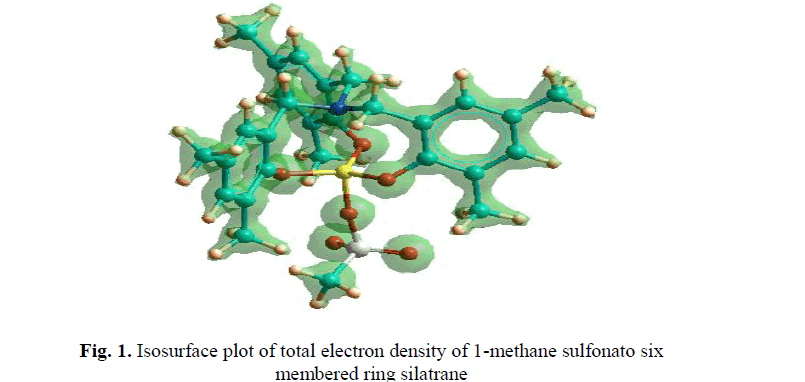
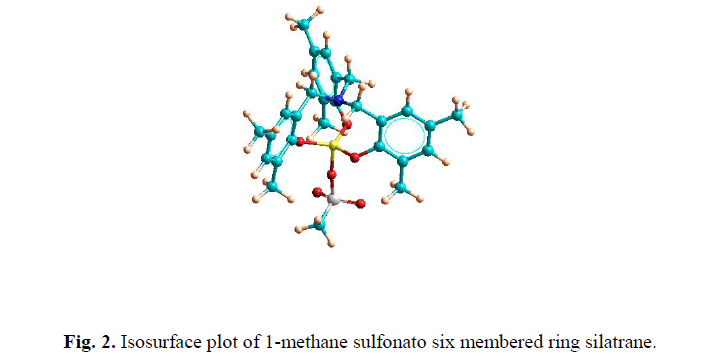
| Isosurface plot of total electron density of 1-methane sulfonato six membered ring silatrane 1 have been depicted in Fig.1 and Fig. 2. The different semi-empirical quantum mechanical calculations predict that transannular Si-N bond distance in five-membered ring silatrane ring is found to be considerably reduced as compared to the six-membered ring silatrane. We found from our previous results of ΔHf of values 1-isothiocyanato six membered ring silatrane that the present methane sulfonato is more stable. The same conclusion is reached for five-membered ring silatrane.This is partially due to ring enlargement and also due to the increased interaction in the transannular Si-N bond. The high negative value of ΔHf indicates the high thermal stability of the synthesized silatranes. These calculations also predict high dipole moment for these silatranes. |
 |
 |
V. CONCLUSIONS |
| CH3(O)2SOSi(OC6H2Me2CH2)3N (1), CH3OSi(OC6H2Me2CH2)3N (2) and CH3(O)2SO.Si(OCH2CH2)3N (3) have been repoeted in good yield. These novel silatranes are valuable from coordination chemistry, biological application and material science point of view. Semi-empirical quantum mechanical calculations predict the dependence of the N→Si transannular bond length on atrane ring and exocyclic substituents. |
References |
|